Subjects: TRI, FDA
Privo Technologies Receives U.S. FDA Orphan Drug Designation
PEABODY, Mass., Aug. 12, 2022 /PRNewswire/ -- Privo Technologies, Inc. ("Privo") announced today that the U.S. Food and Drug Administration (FDA) granted Orphan Drug Designation (ODD) to Privo's PRV platform delivery of cisplatin for the treatment of carcinoma in situ (CIS) of the anterior 2/3 of the oral cavity. This represents Privo's third ODD for a rare disease following their approval in oral cavity cancers and anal cancers. Privo Technologies is a clinical stage biopharmaceutical company that has designed and developed a nanoengineered drug delivery platform to safely deliver highly potent and toxic APIs locoregionally, led by founder and CEO Manijeh Goldberg, PhD.
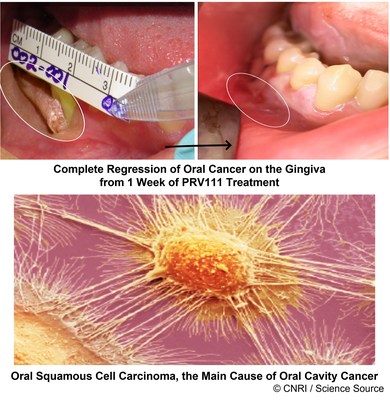
"Achieving this important regulatory milestone means we are one step closer to bringing new treatment options to patients diagnosed with oral CIS. The standard of care currently is surgery, resulting in severe disfigurement and poor quality of life. Our novel transmucosal delivery system with embedded cisplatin loaded nanoparticles, PRV111, allows for patients to be treated with topical chemotherapy providing an alternative to surgery that effectively treats cancer while maintaining the form and function of the oral cavity," said Dr. Goldberg.
FDA Orphan Drug Designation is granted to investigational therapies addressing rare medical diseases or conditions that affect fewer than 200,000 people in the United States. Orphan drug status provides benefits to drug developers that includes assistance in the drug development process, tax credits for clinical costs, exemption from FDA PDUFA fees, and seven years of post-approval exclusivity.
Privo Technologies, Inc. (Privo) is a phase 3 clinical-stage biopharmaceutical company committed to developing novel therapies with the potential to transform standard of care for treating solid tumors by offering safer and more effective alternatives. Privo is headquartered in Peabody, Massachusetts.
Privo's lead asset, PRV111 has been shown to be effective in patients with head and neck cancer in several hospitals across the US during a safety and efficacy Phase I/II clinical study, dramatically reducing tumor volume without any systemic toxicity. For additional information on Privo Technologies, Inc. please visit www.privotechnologies.com.
CONTACT: [email protected]
SOURCE Privo Technologies
These press releases may also interest you
|
News published on and distributed by: