Subject: PDT
Integral Molecular Launches TiterSafetm Influenza Virus-Like Particles for Safe and Convenient Vaccine Testing
PHILADELPHIA, Nov. 7, 2023 /PRNewswire/ -- Integral Molecular, a leading provider of reagents for vaccine evaluation, announces the launch of TiterSafetm influenza virus-like particles, an off-the-shelf virus-alternative for key experiments in testing influenza vaccines. TiterSafe is a safe and convenient substitute for live virus that is ready for immediate use, reduces biosafety hazards for lab workers, and is well-suited for high-throughput assays. In contrast, traditional assays require the difficult preparation of live influenza virus, potentially expose researchers to infection, and need dedicated containment facilities for pandemic virus strains.
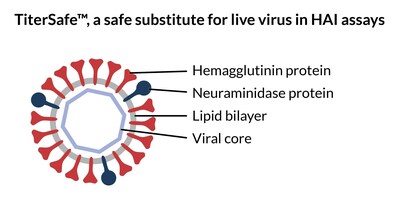
TiterSafe particles mimic the architecture of live virus, featuring surface influenza proteins hemagglutinin (HA) and neuraminidase (NA) along with an interior protein core. Crucially, they lack DNA or RNA components that enable virus replication. The HA protein on TiterSafe particles behaves identically to HA on live virus in experiments, sticking to red blood cells and causing them to clump, or "hemagglutinate". Inhibition of this clumping by anti-HA antibodies in serum (an "HAI assay") is the standard FDA-accepted method for influenza vaccine assessment. TiterSafe comes in assay-ready portions for immediate use in HAI assays and is available with HA and NA variants to match seasonal strains of influenza.
"For over 20 years we have responded to viral threats including SARS-CoV-2, dengue, Zika, and now influenza by developing products that allow any lab to work safely with viruses," said Kyle Doolan, Director of R&D at Integral Molecular. "While the FDA-accepted HAI assay is relatively easy to perform, scaling up reagents and working with highly infectious live viruses requires considerable time and resources. We are pleased to offer this off-the-shelf reagent that will enable vaccine developers to focus their time on more pressing research."
TiterSafe is currently available for seasonal and pandemic influenza strains. Additional products will be released as new strains are announced.
About Integral Molecular
Integral Molecular (integralmolecular.com) is the industry leader in developing and applying innovative technologies that advance the discovery of therapeutics against difficult protein targets. With 20 years of experience focused on membrane proteins and antibodies, Integral Molecular's technologies have been integrated into the drug discovery pipelines of over 500 biotech and pharmaceutical companies to help discover new therapies for cancer, diabetes, autoimmune disorders, and viral threats such as SARS-CoV-2, Ebola, Zika, and dengue viruses.
Follow Integral Molecular on LinkedIn.
Press Contact:
Integral Molecular, Inc.
Soma Banik, PhD, Director of Public Relations
215-966-6061
[email protected]
www.integralmolecular.com
SOURCE Integral Molecular
These press releases may also interest you
|
News published on and distributed by: