Subject: FDA
Magnolia Medical Expands Steripath® Micro Initial Specimen Diversion Device® (ISDD®) Product Family with New FDA 510(k) Clearance
Designed in collaboration with leading adult and children's hospitals, Steripath Micro is the only FDA 510(k)-cleared low-diversion volume device platform with a specific indication to reduce blood culture contamination1
SEATTLE, April 11, 2023 /PRNewswire/ -- Magnolia Medical Technologies, Inc., announced today U.S. Food and Drug Administration (FDA) 510(k) clearance of 19 new Steripath® Micro configurations within the company's ISDD® product portfolio. This clearance provides hospitals with an expansive array of new options, including direct-to-bottle and BD Vacutainer® UltraTouchtm push-button blood collection set configurations, to serve the needs of all patient populations.
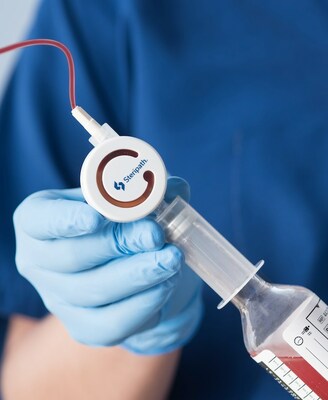
Employing the same clinically proven Initial Specimen Diversion Device® technology as Magnolia Medical's flagship product family of Steripath® Initial Specimen Diversion Devices®, the Steripath Micro device family combines a small, highly intuitive design for optimal user-experience with the speed of traditional blood culture collection methods. Steripath Micro is the only low-diversion volume blood culture collection device family with a specific FDA 510(k)-cleared indication to reduce blood culture contamination.1
All Steripath Micro needle configurations integrate the BD Vacutainer® UltraTouchtm push button blood collection set. UltraTouchtm needles have an ultra?thin?wall cannula, which provides a larger inner diameter while maintaining a true?to?gauge size outer cannula diameter (BD RightGaugetm Technology). Importantly, this technology innovation allows for greater sample fill volume to be collected into blood culture bottles by improving blood flow rates. Additionally, the BD PentaPointtm cannula technology integrated into BD Vacutainer® UltraTouchtm wingsets helps penetrate the skin with greater ease.10
"We are delighted to launch the expanded family of Steripath Micro configurations as an integral part of our Initial Specimen Diversion Device portfolio. We developed the Steripath Micro platform in close collaboration with our customers to ensure the ability to provide improved blood culture accuracy for all patient populations, including those that are most vulnerable," said Greg Bullington, CEO of Magnolia Medical.
"The availability of these configurations will further accelerate our co-selling and co-marketing activities with BD as all Steripath Micro needle configurations come standard with BD's best-in-class UltraTouchtm needle innovation.
"This new product offering demonstrates our continued commitment to providing hospitals and healthcare systems with the best possible solutions that optimally balance ease of use and clinical performance to support achievement of CLSI and CDC's new 1% goal for blood culture contamination rates," Bullington concluded.2,3,4
Steripath Micro was developed in collaboration with clinicians in a variety of hospital settings throughout the country to optimize the blood culture collection process by allowing for fast, easy, and effective diversion and test sample collection. Using an active blood culture bottle- or syringe-driven diversion method, Steripath Micro diverts the initial 0.5 to 1.0 mL of blood into the diversion chamber, preventing potential contaminants from distorting the blood sample to be tested. Once diversion is complete, the clinician simply pushes a button to sequester potential contaminants and automatically open a second, sterile blood flow pathway to collect blood for testing.
In October 2022, BD (Becton, Dickinson and Company) and Magnolia Medical announced a co-exclusive commercial agreement aimed at helping U.S. hospitals reduce blood culture contamination by improving sepsis testing accuracy. The goal of the partnership is to significantly improve patient safety and outcomes while reducing avoidable hospital costs. Under the agreement, BD and Magnolia Medical both co-sell and co-market Magnolia Medical's Steripath® and Steripath® Micro Initial Specimen Diversion Device® platforms, complementing BD's bloodstream infection and specimen collection portfolios, including BD Vacutainer® push button and BD Vacutainer® UltraTouchtm blood collection sets.
As with all solutions across the Steripath Initial Specimen Diversion Device platform, Steripath Micro is backed by Magnolia Medical's market exclusive Clinical Performance Guarantee ? achieve sustained reduction in blood culture contamination of 50% or greater, or your money back.11
About Steripath
The Steripath® Initial Specimen Diversion Device® platform offers the only all-in-one devices that are clinically proven to meet the ENA, INS, CDC, and CLSI evidence-based best practice guidelines to reduce blood culture contamination.2,3,4,5,6 To date, 20 clinical studies have been completed supporting the clinical and cost effectiveness of Steripath. All studies reported sustained contamination rates of 0-1% using Steripath, up to a 31% reduction in vancomycin days of therapy, and as much as a 12-fold decrease in false-positive central line-associated blood stream infections (CLABSIs) over extended periods of time.7,8,9
Steripath has been adopted by hundreds of U.S. hospitals and healthcare systems to address the problem of blood culture contamination, which can lead to sepsis misdiagnosis resulting in unnecessary, prolonged, and harmful antibiotic treatment, extended length of hospital stay, false-positive CLABSIs, and wasted healthcare resources.
About Magnolia Medical Technologies
Magnolia Medical Technologies develops, manufactures, and markets innovative blood and bodily fluid collection devices to facilitate significant improvements in the accuracy, consistency, and predictability of critical laboratory tests. Magnolia Medical invented and patented the Initial Specimen Diversion Technique® (ISDT®) and Initial Specimen Diversion Device® (ISDD®) for blood culture collection and contamination prevention. The company has amassed an intellectual property portfolio, including more than 130 issued method, apparatus, and design patents with more than 50 additional patent applications pending. For more information, visit www.magnolia-medical.com.
- Indicated for use as a blood collection system that diverts and sequesters the initial specimen prior to collection of a subsequent test sample to reduce the frequency of blood culture contamination when contaminants are present in the initial blood sample compared to blood cultures drawn with standard procedures without manual diversion.
- CDC. Blood Culture Contamination Prevention Actions: An Overview of Infection Control and Antibiotic Stewardship Programs Working with the Clinical Laboratory. July 2022.
- CLSI. Principles and Procedures for Blood Cultures. 2nd Ed. CLSI Guideline M47. Clinical and Laboratory Standards Institute; 2022.
- CDC National Email Update to Clinicians. Clinicians: Use this guide to decrease blood culture contamination rates. July 22, 2022.
- Vanhoy MA, Horigan A, Kaiser J, et al. Emergency Nurses Association (ENA). Clinical practice guideline: prevention of blood culture contamination. 2020.
- Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice, 8th edition. J Infus Nurs. 2021 Jan-Feb 01;44(1S Suppl 1): S1-S224.doi: 10.1097/NAN.0000000000000396.
- Date on file.
- Nielsen LE, Nguyen K, Wahl CK, et al. Initial Specimen Diversion Device® reduces blood culture contamination and vancomycin use in academic medical center. J Hosp Infect. 2021;117. doi:https://doi.org/10.1016/j.jhin.2021.10.017.
- Tompkins LS, et al. Getting to zero: impact of a device to reduce blood culture contamination and false-positive central line-associated blood stream infections. Accepted for publication by ICHE on Nov 7, 2022.
- BD data on file. Benchtop test results may not necessarily be indicative of clinical performance.
- Additional terms and conditions apply.
SOURCE Magnolia Medical Technologies
These press releases may also interest you
|
News published on and distributed by: