Subject: SVY
Noster Microbiome Research: Analyzing Bacterial Digest
KYOTO, Japan, Dec. 2, 2022 /PRNewswire/ -- Researchers at Noster Inc. report in Medical Mass Spectrometry a method for the efficient quantification of a set of biomolecules produced by gut bacteria breaking down nutrients. The method has important potential for monitoring the composition of diet-dependent biological samples.
Gut microbiota are the collective of microorganisms ? including bacteria and viruses ? that reside in the digestive tracts of humans and animals. Among the many functions of the gut microbiota is the processing of nutrition that cannot otherwise by digested. Carbohydrates, such as sugars, fibers and starches, for example, require bacterial intervention to be properly broken down. Dietary fats are processed by both digestive enzymes (proteins naturally produced in human and animal bodies) and gut microorganisms; this so-called process of metabolization results in the production of metabolites, small organic molecules having various biological functions. Some of these metabolites are produced by the gut microbiota only. Being able to detect such metabolites with high sensitivity therefore provides a way to assess their significance as well as their therapeutic potential. Now, Kowa Tsuji from Noster Inc. and colleagues under supervision of Dr. Makoto Arita at Keio University, have developed a method enabling a comprehensive analysis of a whole range of metabolites produced solely by the gut microbiota.
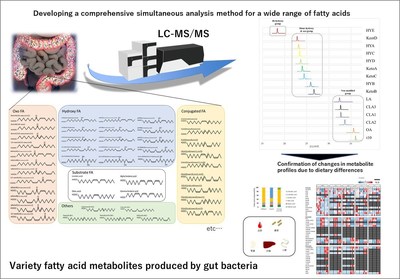
The researchers focused on the metabolites resulting from the breakdown of polyunsaturated fatty acids (PUFAs), which are found in vegetable oils. Gut bacteria convert PUFAs into fatty acid metabolites that cannot be produced by host enzymes. For the detection of these metabolites, Tsuji and colleagues used a technique called liquid chromatography tandem mass spectroscopy (commonly abbreviated as LC-MS/MS), enabling selective and precise concentration measurements of the compounds. An essential part of the work consisted in obtaining synthetic standards of the metabolites ? biogenically prepared compounds with known concentration, with which the LC-MS/MS method could be developed.
The scientists tested their method by analyzing mouse feces; they successfully detected several fatty acid metabolites produced by the gut microbiota. The obtained concentration estimates were in the micromolar range, which is an indication that the method is sensitive enough to monitor natural levels of fatty acid metabolites in biological systems. The researchers also applied the method to tissues obtained from mice subjected to different diets. The obtained metabolite levels showed good correlation with the dietary sources (vegetable oils) of PUFAs.
The method of Tsuji and colleagues enables the efficient detection and quantification of 45 metabolites produced by the gut microbiota, and holds promise for nutritional and medical research. Quoting the scientists: "This analytical method will be improved to aid future studies to elucidate the biological significance of bacteria-derived fatty acid metabolites in controlling host health and diseases."
Background
Liquid chromatography tandem mass spectroscopy (LC-MS/MS)
Liquid chromatography tandem mass spectroscopy (LC-MS/MS) is a technique used for the detection and quantification of chemical compounds in a sample. It combines liquid chromatography, a technique used for physically separating substances, and mass spectroscopy, used for analyzing masses of substances. LC-MS/MS can be used for analyzing a wide range of chemical compounds, including metabolites.
In LC-MS/MS, a sample is first pumped through an adsorbent (stationary phase, the LC column) by a liquid (mobile phase) at high pressure. Chemical interactions between the sample's components, the stationary and the mobile phase cause different migration rates of the sample's components throughout the LC column, which results in their separation. By choosing particular stationary and mobile phase compositions, customized separation can be achieved for a large variety of samples. After the LC step, the LC effluent is fed into a mass spectrometer, which ionizes particles. Ionized particles are deflected by means of an electromagnetic field; the amount of deflection depends on their mass-to-charge ratio.
Kowa Tsuji from Noster Inc. and colleagues now developed a LC-MS/MS procedure enabling the comprehensive analysis of 45 metabolites derived from polyunsaturated fatty acids (PUFAs).
Reference
Kowa Tsuji, Wataru Shimada, Shigenobu Kishino, Jun Ogawa, and Makoto Arita. Comprehensive analysis of fatty acid metabolites produced by gut microbiota using LC-MS/MS-based lipidomics, Medical Mass Spectrometry 6 (2022).
DOI: 10.24508/mms.2022.11.003
URL: http://www.jsbms.jp/english/publish/03_04_Reserch-Paper_Tsuji_et_al_4th_sawa.pdf
Contact details
Noster Inc., International Relations,
35-3 Minamibiraki, Kamiueno-cho,
Muko-shi, Kyoto, 617-0006, Japan
E-Mail: [email protected]
Telephone: 81 (0)-75-921-5303
Noster website
https://www.noster.inc/
Noster's analytical services
https://www.noster.inc/services/
Photo: https://mma.prnewswire.com/media/1960554/Noster_Microbiome_research.jpg
SOURCE Noster Inc
These press releases may also interest you
|
News published on and distributed by: