Subjects: CHI, FDA, DEI
PCA 500-- the world's most efficient and versatile 12-lead ECG, just received FDA clearance for pediatric use
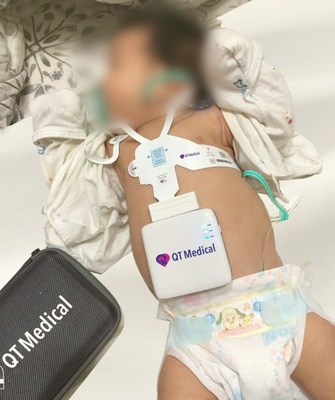
LOS ANGELES, Oct. 4, 2022 /PRNewswire/ -- QT Medical announces FDA clearance of PCA 500, a resting 12-lead electrocardiogram (ECG or EKG), for use in pediatric patients. First cleared in 2018 for professional and personal use by adults 18 years and older, PCA 500's new FDA clearance expands its indication for use to all pediatric populations, including: newborns, infants, children and adolescents.
The development of PCA 500's pre-positioned electrodes and compact recorder was initially funded by NIH for an easy-to-use ECG specifically designed for pediatric use. PCA 500 offers digital, mobile and cloud-based ECG management solution and is widely used by airlines, telehealth practices, clinics, urgent care centers, skilled nursing facilities, hospitals, schools, and in clinical trials, with many CRO partners anticipating the pediatric clearance. QT Medical will introduce PCA 500 to the pediatric market at the 2022 American Academy of Pediatrics National Conference.
"The potential of PCA 500 in improving the heart health of children is extremely exciting. As a pediatric cardiologist, I know how difficult it is to get an ECG on a child. With PCA 500, we can make ECG technology easily accessible to all children. We believe this will make a difference in many lives, which is the exact reason why I founded QT Medical." said Dr. Ruey-Kang Chang, CEO of QT Medical, Inc.
Two new initiatives will be announced at the pediatric market launch of PCA 500. First, Youth Xpress ECG, in partnership with Who We Play For, a non-profit organization dedicated to preventing sudden cardiac arrest of young people, is a mail delivery home 12-lead ECG testing service with interpretation by expert pediatric cardiologists. Second, Baby Xpress ECG, an at-home ECG screening service for newborns and infants with increased risks for long QT syndrome (LQTS). With Baby Xpress ECG, when a baby's ECG shows prolonged QT interval, a saliva test will be used to check for genetic mutations known for causing LQTS. Long QT syndrome, occurring 1 in every 2000 babies, is a known cause for sudden death (including SIDS).
About QT Medical
QT Medical is a medtech company with a focus on high quality 12-lead diagnostic ECG for use by healthcare professionals and patients. With its simplicity, ease of use, mobile technology and cloud management, PCA 500 brings hospital-grade ECG to millions of patients for better cardiac care at home. More information at: www.qtmedical.com
SOURCE QT Medical, Inc.
These press releases may also interest you
|
News published on and distributed by: