Subjects: MAT, IMA
Benuvia, a Drug Developer Focused on Pharmaceutical Cannabinoids, and the owner of FDA Approved SYNDROS®, Receives Approval from the US DEA to Manufacture Psychedelic Active Pharmaceutical Ingredients including Psilocybin, MDMA and DMT
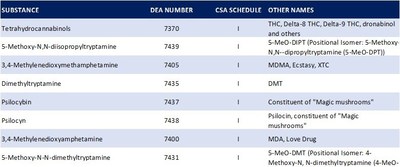
- With this approval, Benuvia has begun focusing on four primary psychedelic ingredients: Psilocybin, N,N-Dimethyl-5-Methoxy-Tryptamine (5-MeO DMT), Dimethyltryptamine (DMT), and 3,4-Methylenedioxymethamphetamine, or MDMA
- Benuvia will manufacture psychedelic APIs in its 83,000 square foot manufacturing facility that is permitted by the US DEA for Schedule I to III Controlled Substances, and is FDA registered and a cGMP facility
- Benuvia is positioning itself to become a leading global supplier of psychedelic active pharmaceutical ingredients (APIs), complementing its established portfolio of cannabinoid APIs
ROUND ROCK, Texas, May 16, 2022 /PRNewswire/ -- Benuvia, Inc., a drug developer and manufacturer of APIs focused on cannabinoids, with a growing portfolio of drug products and intellectual property, that has previously announced the signing of a definitive agreement with Pono Capital Corp (Nasdaq: PONOU), announced today that it has added to its expanding psychedelic manufacturing capabilities by adding a new set of Schedule I controlled substances to its U.S. Drug Enforcement Administration Bulk Manufacturing registration under the U.S. Controlled Substance Act (CSA). With this approval, Benuvia has begun focusing on four primary psychedelic ingredients: Psilocybin, N,N-Dimethyl-5-Methoxy-Tryptamine (5-MeO DMT), Dimethyltryptamine (DMT), and 3,4-Methylenedioxymethamphetamine, or MDMA.
In addition to its drug development business, Benuvia offers cannabinoid and psychedelic contract development and manufacturing services for APIs and drug products and has an industry-leading track record of producing cannabinoid APIs under commercial drug master files (DMFs) that are referenced in approved pharmaceutical New Drug Applications. Benuvia operates in full compliance with global pharmaceutical regulatory standards and utilizes its DMFs as well as extensive regulatory experience to aid pharmaceutical companies in gaining regulatory approval of new and improved therapies to treat, cure, or prevent diseases.
"We are excited to announce our approval by the DEA to manufacture Psilocybin, MDMA and DMT for emerging providers of psychedelic-based drugs focused on neural and mental illness indications," said Joe Shupp, Chief Commercial Officer. "As pharmaceutical companies become more aware of the positive effects of cannabinoids and psychedelic compounds for therapeutic use, the demand for APIs is expected to grow. Our diversified portfolio of APIs and entry into the development of novel psychedelic compounds aims to strengthen Benuvia's position as a drug developer and provider of APIs focused on cannabinoids and psychedelics."
This approval will also enable Benuvia to advance its life cycle management services to drug developers for their psychedelic development programs. Benuvia intends to develop novel intellectual property formulations of these psychedelic compounds, and will provide full toxicology, chemistry, manufacturing and controls, or CMC, and full data analysis. In addition, Benuvia, through its partners, intends to manage all research and development, manufacturing, analytical methods, intellectual property capture, placebo manufacture, randomization, and assistance with clinical trials. This will enable Benuvia to create custom technologies that allow rapid and cost-effective scale up of commercial operations for production in anticipation of research success.
Benuvia is positioned to become a global supplier of cannabinoid APIs, complemented by its emerging portfolio of psychedelic APIs. As it develops long-term partnerships with developers and suppliers, leveraging its broad platform of development, formulations, and process technologies for life cycle management of drug development and APIs, Benuvia will enable its partners to expediate the development and production of their drug products.
About Benuvia, Inc.
Benuvia, Inc. is a drug developer and manufacturer of APIs focused on cannabinoids and psychedelics, with a growing portfolio of drug products and intellectual property. Benuvia owns the FDA approved cannabinoid drug SYNDROS® (dronabinol oral solution CII). SYNDROS® is FDA approved for chemotherapy-induced nausea and vomiting ("CINV"), in adult cancer patients who have failed to respond adequately to conventional anti-nausea medicines, and loss of appetite (anorexia) in adult patients with acquired immune deficiency syndrome ("AIDS"). Benuvia is pursuing the 505(b)(2) regulatory pathway with the FDA for Investigational New Drugs for its dronabinol oral solution, with a focus on large opportunities that have significant unmet needs with industry research and studies supporting targeted efficacy endpoints. Benuvia manufactures APIs in its 83,000 square foot cannabinoid and psychedelic manufacturing facility that is permitted by the US DEA for Schedule I to III Controlled Substances, is FDA registered and a cGMP facility. Benuvia has a robust portfolio of patents and patents pending and is pursuing new intellectual properties for its drug products.
About Pono Capital Corp
Pono Capital Corp is a blank check company formed for the purpose of effecting a merger, share exchange, asset acquisition, stock purchase, reorganization, or similar business combination with one or more businesses. In August 2021, Pono Capital Corp consummated a $116 million initial public offering of 11.6 million units (reflecting the underwriters' exercise of their over-allotment option in full), each unit consisting of one of the Company's Class A ordinary shares and three-quarters of one warrant, each whole warrant enabling the holder thereof to purchase one Class A ordinary share at a price of $11.50 per share. Pono Capital Corp's securities are quoted on Nasdaq under the ticker symbols PONOU, PONO and PONOW.
Additional Information and Where to Find It
Pono Capital Corp intends to file with the SEC a registration statement on Form S-4 with a proxy statement containing information about the proposed transaction and the respective businesses of Benuvia and Pono Capital Corp. Pono Capital Corp will mail a final prospectus and definitive proxy statement and other relevant documents after the SEC completes its review. Pono Capital Corp stockholders are urged to read the preliminary prospectus and proxy statement and any amendments thereto and the final prospectus and definitive proxy statement in connection with the solicitation of proxies for the special meeting to be held to approve the proposed transaction, because these documents will contain important information about Pono Capital Corp, Benuvia, and the proposed transaction. The final prospectus and definitive proxy statement will be mailed to stockholders of Pono Capital Corp as of a record date to be established for voting on the proposed transaction. Stockholders of Pono Capital Corp will also be able to obtain a free copy of the proxy statement, as well as other filings containing information about Pono, without charge, at the SEC's website (www.sec.gov) or by calling 1-800-SEC-0330. Copies of the proxy statement and Pono Capital Corp's other filings with the SEC can also be obtained, without charge, by directing a request to: [email protected]. The information contained in, or that can be accessed through, Benuvia's website is not incorporated by reference in, and is not part of, this press release.
No Offer or Solicitation
This press release does not constitute (i) a solicitation of a proxy, consent, or authorization with respect to any securities or in respect of the proposed business combination, or (ii) an offer to sell or the solicitation of an offer to buy any securities, or a solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of the U.S. Securities Act.
Participants in the Solicitation
Benuvia and Pono Capital Corp and their respective directors and officers and other members of management and employees may be deemed participants in the solicitation of proxies in connection with the proposed business combination. Pono Capital Corp stockholders and other interested persons may obtain, without charge, more detailed information regarding directors and officers of Pono Capital Corp in Pono Capital Corp's initial public offering prospectus, which was declared effective the SEC on August 10, 2021. Information regarding the persons who may, under SEC rules, be deemed participants in the solicitation of proxies from Pono Capital Corp's stockholders in connection with the proposed business combination will be included in the definitive proxy statement/prospectus the Pono Capital Corp intends to file with the SEC.
Caution Concerning Forward-Looking Statements
Certain statements herein are "forward-looking statements" made pursuant to the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Actual results may differ from their expectations, estimates, and projections and, consequently, you should not rely on these forward-looking statements as predictions of future events. In some cases, you can identify forward-looking statements through the use of words or phrases such as "may", "should", "could", "predict", "potential", "believe", "will likely result", "expect", "continue", "will", "anticipate", "seek", "estimate", "intend", "plan", "projection", "would" and "outlook", or the negative version of those words or phrases or other comparable words or phrases of a future or forward-looking nature, but the absence of such words does not mean that a statement is not forward-looking. These forward-looking statements are not historical facts and are based upon estimates and assumptions that, while considered reasonable by Pono Capital Corp and its management, and Benuvia and its management, as the case may be, are inherently uncertain. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: (1) the occurrence of any event, change or other circumstances that could give rise to the termination of negotiations and any subsequent definitive agreements with respect to the proposed business combination; (2) the outcome of any legal proceedings that may be instituted against Pono Capital Corp, Benuvia, the combined company or other following the announcement of the proposed business combination and any definitive agreements with respect thereto; (3) the inability to complete the proposed business combination due to the failure to obtain approval of the stockholders of Pono Capital Corp, to obtain financing to complete the proposed business combination or to satisfy other conditions to closing; (4) changes to the proposed structure of the proposed business combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the proposed business combination; (5) the ability to meet stock exchange listing standards following the consummation of the proposed business combination; (6) the risk that the proposed business combination disrupts current plans and operations of Pono Capital Corp or Benuvia as a result of the announcement and consummation of the proposed business combination; (7) the ability to recognize the anticipated benefits of the proposed business combination, which may be affected by, among other things, competition and the ability of the combined company to grow and manage growth profitably, maintain relationships with customers and retain its management and key employees; (8) costs related to the proposed business combination; (9) changes in applicable laws or regulations and delays in obtaining, adverse conditions contained in, or the inability to obtain regulatory approvals required to complete the proposed business combination; (10) Benuvia's estimates of expenses and profitability and underlying assumptions with respect to stockholder redemptions and purchase price and other adjustments; (11) Benuvia's inability to market its existing drug and develop new drugs for FDA approval; (12) the addressable market Benuvia intends to target does not grow as expected; (13) increased regulatory costs and compliance requirements in connection with drug development; (14) Benuvia's inability to expand and diversify its manufacturing customer base; (15) the loss of any key executives; (16) the loss of any relationships with key partners; (17) the loss of any relationships with key suppliers; (18) the inability to protect Benuvia's patents and other intellectual property; (19) lower than expected adoption rates for SYNDROS®; (20) new FDA approved drugs that compete with Benuvia in targeted indications; (21) the inability to initiate and increase engagement with distributors; (22) fluctuations in results of Benuvia's major manufacturing customers; (23) Benuvia's ability to execute its business plans and strategy; (24) Benuvia's ability to maintain sufficient inventory and capacity to meet customer demand; (25) Benuvia's inability to deliver expected cost and manufacturing efficiencies; (26) general economic conditions and geopolitical uncertainty; and (27) other risks and uncertainties indicated from time to time in other documents filed or to be filed with the SEC by Pono Capital Corp. See "Risk Considerations" in the corporate presentation, which will be provided in a Current Report on Form 8-K to be filed by Pono Capital Corp with the SEC and available at www.sec.gov.

Photo - https://mma.prnewswire.com/media/1816110/BenuviaTable.jpg
Logo - https://mma.prnewswire.com/media/1815918/BenuviaInc_Logo_Logo.jpg
These press releases may also interest you
|
News published on and distributed by:



