Subject: FDA
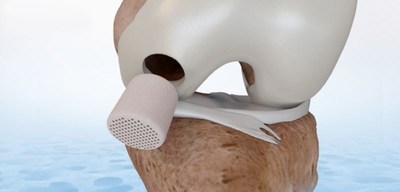
FDA approves CartiHeal's Implant for the Treatment of Cartilage and Osteochondral Defects
KFAR SABA, Israel, March 30, 2022 /CNW/ -- CartiHeal Ltd, developer of implants for the treatment of cartilage and osteochondral defects in arthritic and non-arthritic knee-joints, today announced that the U.S. Food and Drug Administration (FDA) has granted Premarket Approval (PMA) for its Agili-Ctm implant.
The implant is indicated for the treatment of an International Cartilage Repair Society (ICRS) grade III or above knee-joint surface lesion(s), with a total treatable area of 1-7cm2, without severe osteoarthritis (Kellgren-Lawrence grade 0-3).
PMA approval was granted based on the results of a two-year IDE pivotal clinical study. The study confirmed superiority of the Agili-Ctm implant over the current Surgical Standard of Care (SSOC) ? microfracture and debridement, for the treatment of knee joint surface lesions, chondral and osteochondral defects. The study was multicenter, 2:1 randomization, open-labeled and controlled. A total of 251 subjects were enrolled, 167 in the Agili-Ctm arm, and 84 in the SSOC arm, in 26 sites both in and outside the US.
The primary endpoint of the study was the change from baseline to 24 months in the average Knee injury and Osteoarthritis Outcome Score (KOOS Overall), which consists of 5 subscales: Pain, Other Symptoms, Quality of Life (QOL), Activities of Daily Living (ADL) and Sports. The KOOS Overall Score ranges from 0 to 100, where higher values represent better outcomes.
The data generated from the trial demonstrated superiority of Agili-Ctm to the current surgical standard of care (debridement or microfracture, SSOC). The Bayesian posterior probability of superiority after 24 months was determined to be 1.000, exceeding the prespecified threshold of 0.98 required to demonstrate superiority.
- The baseline KOOS Overall score was similar in both groups: 41.2 in the Agili-Ctm arm and 41.7 in the SSOC arm. At Month 24, the KOOS Overall improved to 84.3 in the Agili-Ctm arm compared to 62.0 for the SSOC arm.
- The degree of improvement for Agili-Ctm compared to SSOC was similar for subjects with Mild-moderate Osteoarthritis (Kellgren-Lawrence Grades of 2 or 3) and for subjects with Large Lesions (total lesion areas larger than 3 cm2).
- Superiority of the Agili-Ctm implant over SSOC was confirmed also in all the study's secondary endpoints Confirmatory Endpoints: KOOS Pain, KOOS ADL and KOOS QOL and Responder Rate.
- The responder rate, which was a-priori defined as improvement of at least 30 points in Overall KOOS at 24 months compared to baseline, was 77.8% in the Agili-Ctm arm compared to 33.6% in the SSOC arm.
"The 2-year study results, which demonstrated superiority of the Agili-Ctm implant over the current surgical standard of care, offers an important potential benefit to millions of patients", said Nir Altschuler, CartiHeal's founder and CEO. "This milestone achievement was made possible due to the support of our regulatory advisors, Hogan Lovells, our statistical consultants, Biomedical Statistical Consulting, and the many dedicated investigators and patients who participated in our studies. We are grateful for all their help. FDAs approval enables us to initiate commercialization and provide a superior solution for patients compared to the current standard of care options."
About CartiHeal
CartiHeal, a privately-held medical device company headquartered in Israel and New Jersey, develops proprietary implants for the treatment of cartilage and osteochondral defects in traumatic and osteoarthritic joints.
For more information:
www.cartiheal.com
[email protected]
Photo - https://mma.prnewswire.com/media/1776649/CartiHeal_Implant.jpg
Logo - https://mma.prnewswire.com/media/1776650/CartiHeal_Logo.jpg
SOURCE CartiHeal
These press releases may also interest you
|
News published on and distributed by: