Subject: FDA
/C O R R E C T I O N -- Fifth Eye Inc/
In the news release, Fifth Eye Receives FDA Clearance to Market AHI Systemtm, issued 31-Jan-2022 by Fifth Eye Inc over PR Newswire, we are advised by the company that the subhead should read "hemodynamic instability" rather than "hemodynamic stability" as originally issued inadvertently. The complete, corrected release follows:
Fifth Eye Receives FDA Clearance to Market AHI Systemtm
Second generation medical device software leverages predictive analytics to identify patients at increased risk of future episodes of hemodynamic instability with an ECG signal alone
ANN ARBOR, Mich., Jan. 31, 2022 /PRNewswire/ -- Fifth Eyetm, a provider of intuitive real-time clinical analytics, today announced the FDA provided clearance to market its second generation clinical decision support software, the AHI Systemtm, to hospitals in the United States.
AHI (pronounced 'AH-hee') stands for Analytic for Hemodynamic Instability. Hemodynamic instability is a condition in which blood flow to vital organs is insufficient. Hemodynamic instability can occur suddenly. When left unnoticed or untreated, it is a known cause of significant morbidity and mortality in critically ill or injured patients. AHI, Fifth Eye's first analytic, can detect hemodynamic instability in real-time from information embedded in an ECG signal alone.
The release of the AHI System brings a wealth of enhancements including a new analytic, the AHI Predictive Index (AHI-PI). AHI-PI can automatically and continuously predict the likelihood of future episodes of hemodynamic instability earlier than is possible with vital signs. Early awareness of emerging problems provides clinicians with precious time that may facilitate early intervention to mitigate or avoid a crisis.
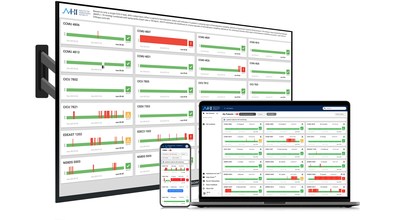
The AHI System provides at-a-glance awareness of patient risk with "traffic-light simplicity" by displaying a red, yellow or green indicator on a multi-patient screen. Clinicians can access AHI through any browser-enabled device, including a mobile phone or tablet. And since data is automatically collected and updated every two minutes, AHI reduces the surveillance burden on the nursing staff while providing access to new and valuable information to help them confidently prioritize their time.
"Hospitals and their staff are being stretched to their limits with over-crowded emergency rooms and ICUs," said Jen Baird, CEO, Fifth Eye. "The AHI System provides physicians and nurses current, clinically validated insights regarding which patients may require additional vigilance to avoid an impending crisis. Conversely, AHI can bring the confidence of objective information to support the timely discharge or transfer of patients to lower acute settings, freeing up precious resources for additional patients."
In the FDA-reviewed clinical study, AHI-PI significantly differentiated critical care patients' likelihood of developing hemodynamic instability. Patients with red high-risk indicators were 51x more likely than those with green low-risk indicators to have an episode of hemodynamic instability in the next hour. Additionally, AHI-PI high-risk indicators predicted 89% of first episodes of hemodynamic instability with a median lead-time of 48 minutes ahead of continuous arterial line blood pressure and heart rate vital signs.
AHI System software is intended for use by healthcare professionals managing in-hospital patients 18 years or older who are receiving continuous physiological monitoring with ECG. AHI surveillance may be initiated on patients monitored with bedside, telemetry or wearable patch ECG and standard electrodes.
About Fifth Eye Inc.
Fifth Eye Inc. is an Ann Arbor, Michigan-based company that develops intuitive, real-time clinical analytics based on physiologic waveforms to improve outcomes and reduce costs. Fifth Eye's machine-learning technology is licensed from the University of Michigan. The AHI Systemtm is FDA cleared, clinical decision support software that monitors hospital patients and continuously predicts the risk of hemodynamic instability earlier than is possible with vital signs. The AHI System requires only the information embedded in an ECG signal. For more information, please visit www.fiftheye.com.
Media Contact:
Patty Keiler
[email protected], (312)550-5394
SOURCE Fifth Eye Inc
These press releases may also interest you
|
News published on and distributed by: