Subject: FDA
SoftSmile Announces FDA 510(k) Clearance for AI Powered Software VISION
NEW YORK, Jan. 18, 2022 /PRNewswire/ -- SoftSmile, a leading medical technology company based in NY, and developer of advanced orthodontic software solutions, announced its software device was cleared by the U.S. Food and Drug Administration (FDA) pursuant to a 510(k) pre-market notification.
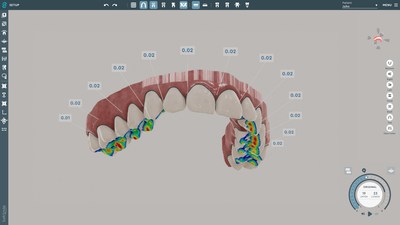
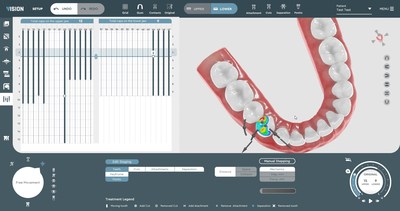
SoftSmile's software VISION, an end-to-end treatment planner made by doctors for doctors, has been granted FDA clearance. This innovative, user-friendly interface gives doctors the ability to create precise aligner treatment plans which are then manufactured using advanced 3-D printing technologies. This news-worthy FDA clearance paves the road to market VISION to doctors and opens the possibility of transforming the existing orthodontic market.
SoftSmile is currently working with established aligner manufacturers. With this FDA clearance, SoftSmile can start marketing Vision as a standalone application to be used directly by doctors.
This FDA clearance sets in motion SoftSmile's plans to transform the market by offering doctors treatment planning software directly, which makes it possible to offer more options to patients thereby cutting costs and increasing efficiency. This development clears the way for this one of a kind innovative software solution with capabilities including but not limited to an automated segmentation feature and intuitive and comprehensive staging. The software automatically generates key aspects of a digitally optimized treatment plan.
VISION's revolutionary technology can now be used in any practice without a doctor having to go through third party designers and/or manufacturers.
"FDA clearance is a huge step for SoftSmile because it establishes Vision's position as a leading software service that complies with all requirements imposed by FDA and is safe for use in the medical practice. There are just a few orthodontic treatment planners that have obtained FDA clearance. SoftSmile's new status is a groundbreaking assurance for the market because the FDA is the best quality check for doctors and patients. This achievement opens new opportunities for the company and proves that our team can solve any type of challenge. It took nearly 18 months, enormous resources, and the coordinated efforts of our engineers and lawyers to be granted FDA clearance. Fortunately, we have only the best at SoftSmile which has now been given the FDA's stamp of approval," said Khamzat Asabaev, Founder and CEO.
About SoftSmile, Inc.
SoftSmile is a New York-based innovation technology company that helps orthodontists to deliver custom, high-quality, and affordable treatment to their patients. Established in 2019, SoftSmile designs and develops an advanced, AI-driven orthodontic software package that applies innovative machine learning technology with sound biomechanical and mathematical principles in a user-friendly interface, giving orthodontists the ability to create aligners treatment plans in-house. These products give orthodontists unparalleled control and precision of the treatment they deliver to their patients. SoftSmile was created by doctors, for doctors.
For additional information about VISION, Inc. please visit https://www.softsmile.com.
CONTACT: [email protected]
SOURCE SoftSmile
These press releases may also interest you
|
News published on and distributed by: