Subject: VEN
Filterlex Medical Closes a $6Million Series A1 Investment to Accelerate the CAPTIS® Cardiovascular Clinical Program
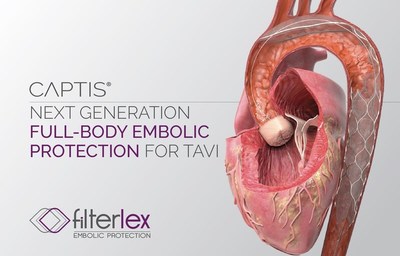
CAESAREA, Israel, Nov. 18, 2021 /PRNewswire/ -- Filterlex Medical, a cardiovascular medical device startup, announced today that it has recently completed a US $6 Million series A1 investment round from leading private healthcare investors. The financing will accelerate implementation of the company's CAPTIS® clinical program. The CAPTIS is a next-generation, full-body embolic protection device to reduce the risk of stroke and other complications during left-heart procedures.
During catheter-based procedures, such as Transcatheter Aortic Valve Replacement (TAVR), embolic particles are often released into the blood flow and may cause neurological deficiencies, from cognitive impairment to debilitating stroke. As demonstrated in our first-in-human study, the CAPTIS device is easily and intuitively deployed and retrieved. It is securely positioned in the aorta, protecting its surface, while facilitating a seamless TAVR procedure. The CAPTIS uniquely requires no additional arterial access and does not interfere with the procedure workflow.
The financing will accelerate implementation of the Company's clinical program. The CAPTIS first-in-human study is successfully ongoing and actively enrolling patients in two leading centers in Israel, Wolfson Medical Center and Rabin Medical Center (Beilinson Hospital). Prof. Haim Danenberg, the study principal investigator and Head of Interventional Cardiology at the Wolfson Medical Center, said "We are thrilled with the opportunity to use the CAPTIS device to protect our TAVR patients' brain and kidneys." Prof. Ran Kornowski, Director of the Cardiology Center at Rabin Medical Center, added, "Our initial experience with the CAPTIS is very positive. The system is safe, intuitive, and easy to use."
Sigal Eli, Founder and CEO, commented: "We are excited by the rapid closing of the new round of funding. With the excellent progress in our clinical program and the recent move to our larger facility in Caesarea, we are well positioned to deliver our vision of making the CAPTIS a best-in-class device."
About Filterlex
Filterlex Medical Ltd. is a cardiovascular medical device startup developing the CAPTIS®, a full-body embolic protection device. In 2016, Filterlex joined Alon MedTech Ventures incubator, owned by Dr. Shimon Eckhouse, a leading entrepreneur and investor in the field of medical devices. The company's founders have vast clinical knowledge and extensive experience in medical device development, commercialization, and marketing. For more information, please visit www.filterlex.com.
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 881076
Press Contact
Sigal Eli
CEO
Filterlex Medical Ltd.
[email protected]
Photo - https://mma.prnewswire.com/media/1691494/Filterlex_Medical.jpg
SOURCE Filterlex Medical
These press releases may also interest you
|
News published on and distributed by: