Subjects: RCL, CFG
Advisory - One lot of the hypoglycemia treatment Glucagon recalled as it may pose serious health risks
OTTAWA, Sept. 25, 2021 /CNW/ -
Summary
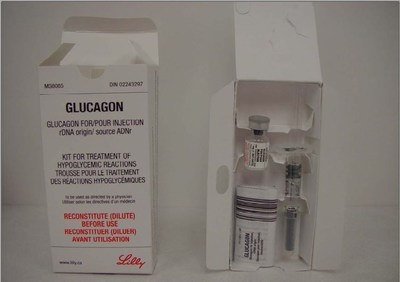
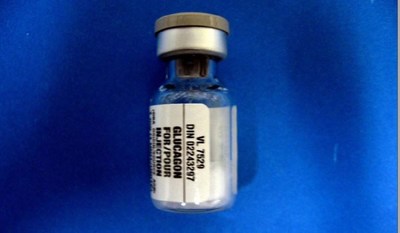
Product: Glucagon (DIN 02243297, lot D239382A, expiry May 10, 2022)
Issue: Eli Lilly Canada Inc. is recalling one lot of Glucagon, following a complaint that a vial from this lot was found to be in liquid form instead of powder form. Glucagon normally comes in a powder form with accompanying diluting solution and should be used immediately after mixing.
What to do: Do not use product from this lot. Call 9-1-1 immediately if you or a family member, including children, have taken this product and are experiencing medical issues, such as hypoglycemia (low blood sugar) or seizures. Consult with your doctor or pharmacist if you have any questions or concerns about the product you are taking.
Issue
Eli Lilly Canada Inc. is recalling one lot of Glucagon (D239382A, expiry May 10, 2022), following a complaint that a vial from this lot was found to be in liquid form rather than powder form. Glucagon normally comes in a powder form with accompanying diluting solution, and should be used immediately after mixing. Other vials in the lot may be affected.
Vials from the affected lot may not work as intended in treating patients with hypoglycemia (low blood sugar) resulting in persisting hypoglycemia. It could also cause other serious health consequences, such as unconsciousness and seizures.
Glucagon is a prescription drug administered by injection for the emergency treatment of severe hypoglycemia (unconsciousness due to low blood sugar), which may occur in diabetic patients (children and adults) treated with insulin.
Health Canada is monitoring the effectiveness of the company's recall and the implementation of any necessary corrective and preventative actions.
What consumers should do
- Call 9-1-1 immediately if you or your child have taken this product and are experiencing medical issues, such as hypoglycemia or seizures.
- Do not use Glucagon from the Lot D239382A.
- Contact Eli Lilly Canada Inc. at 1-888-545-5972 if you have questions about the recall.
- Report any health product-related adverse reactions or complaints to Health Canada.
Affected products
Company | Product Name/Active Pharmaceutical Ingredient (API) | DIN | Lot | Expiry |
Eli Lilly | Glucagon | 02243297 | D239382A | May 10, 2022 |
Photos
Également disponible en français
SOURCE Health Canada
These press releases may also interest you
|
News published on and distributed by: