Subjects: RCL, PSF, CFG
Advisory - BlackOxygen Organics recalls fulvic acid tablets and powder due to potential health risks
OTTAWA, ON, Sept. 23, 2021 /CNW/ -
Summary
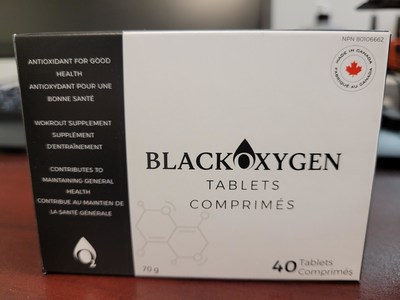
- Product: BlackOxygen Tablets (NPN 80106662) and BlackOxygen Organics Powder (NPN 80097385) (licensed to NuWTR).
- Issue: BlackOxygen Organics is recalling all lots of these products due to potential health risks which may be higher for children, adolescents, and pregnant or breastfeeding women. The products are being promoted in ways and for uses that have not been evaluated and authorized by Health Canada.
- What to do: Stop taking these products. Do not administer the products to children or adolescents. If you or your child or adolescent have taken either of these products and have health concerns, speak to a healthcare provider.
Issue
BlackOxygen Organcs is recalling all lots of BlackOxygen Tablets (NPN 80106662) and BlackOxygen Organics Powder (NPN 80097385) due to potential health risks.
These products are being marketed as fulvic acid supplement(s); however, this use and the quantity of fulvic acid provided by these products has not been evaluated or authorized by Health Canada.
There is limited information to support the safety of fulvic acid when consumed at the quantities found in these products, especially by children and adolescents, and by pregnant or breastfeeding women. The safety of long-term use is also unknown.
The powder product is also being marketed for use on the skin, which has not been evaluated by Health Canada.
The company has stopped sale and is recalling these products. Health Canada is monitoring the recall. If additional safety information
Affected products
- BlackOxygen Tablets (NPN 80106662)
- BlackOxygen Organics Powder (NPN 80097385)
What you should do
- Stop taking these products. Do not administer the products to children or adolescents.
- If you or your child or adolescent have taken either of these products and have health concerns, speak to a healthcare provider.
- Contact BlackOxygen Organics if you have questions about the recall by email at [email protected].
- Report any health product-related side effects or complaints to Health Canada.
Images
BlackOxygen Tablets (NPN 80106662)
BlackOxygen Organics Powder (NPN 80097385)
Également disponible en français
SOURCE Health Canada
These press releases may also interest you
|
News published on and distributed by: