Subject: RCL
BD Announces the Voluntary Recall of Specified Lots of ChloraPreptm Hi-Lite Orangetm 26 mL Applicator in the United States and U.S. Territories Due to Defective Applicator
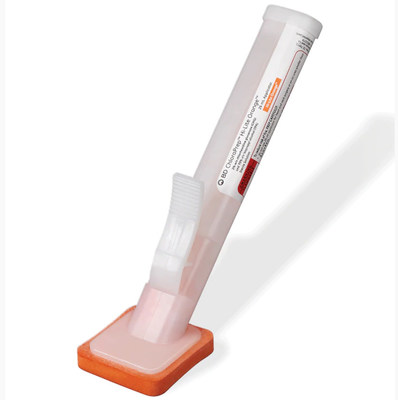
FRANKLIN LAKES, N.J., April 20, 2021 /PRNewswire/ -- BD (Becton, Dickinson and Company), a leading global medical technology company, is voluntarily recalling specified lots of the ChloraPreptm Hi-Lite Orangetm 26 mL Applicator (2% w/w chlorhexidine gluconate (CHG) and 70% v/v isopropyl alcohol (IPA)) to the user level due to a defective applicator. The product is used as an antiseptic for the preparation of the patient's skin prior to surgery to help reduce bacteria that potentially can cause skin infection1.
The ChloraPreptm Hi-Lite Orangetm 26 mL Applicator contains glass ampules that house the sterile ChloraPreptm solution. In normal circumstances, the product is activated by squeezing the wing on the applicator to break the ampule, which releases the solution to the sponge head for application to the patient's skin. In certain lots, the applicator end cap was improperly secured due to a manufacturing error. This can result in broken glass and solution dropping out of the applicator once activated. In some cases, the glass ampules can drop out before activation and shatter if striking a hard surface, resulting in solution and glass fragments scattering in the procedure area and potentially causing injury to patients and health care professionals. These products were distributed in the United States and Puerto Rico.
BD Risk Assessment
Immediate health consequences could be lacerations to patient and/or user of the device. It is possible that these could range from superficial to deep lacerations. While BD has no current reports of severe injury, it is possible that lacerations could cause damage to structures such as nerves or tendons, however, this is considered unlikely. Long-term health consequences could include superficial or deep lacerations that could lead to infection and scarring. "Flying" glass shards could potentially cause injury to the patient or user, including eye injuries. Splashing ChloraPreptm solution could contact the eyes of the user or patient. If tissues or organs other than the skin and subcutaneous tissues are damaged, permanent impairment could occur. While blood loss could accompany these injuries, it would be readily controllable with simple standard measures. To date, BD has received 56 complaints with only one laceration injury reported with respect to this issue.
As part of the voluntary recall to the user level, the company is notifying customers and distributors affected by the recall. BD's Customer Recall Notification provides instructions to customers and distributors for disposal and replacement of the impacted ChloraPreptm Hi-Lite Orangetm 26 mL Applicator (see list of affected lot numbers below).
Catalog No. | Product | Lot Number | Expiration Date |
930815 | BD ChloraPreptm Hi-Lite Orangetm 26mL Applicator | 0108186 | 04/30/2023 |
0327867 | 11/30/2023 | ||
0327868 | |||
0328213 | |||
0328947 | |||
0328949 | |||
0329475 | |||
0329477 | |||
0330457 | |||
0330606 | |||
0330955 | |||
0333826 | |||
0333852 | |||
0333855 | |||
0334119 | |||
0335029 | |||
0335787 | |||
0335792 | |||
0336051 | |||
0336506 | |||
0336972 | |||
0337025 | |||
0337245 | |||
0338542 | |||
0338653 | |||
0338656 | |||
0338852 | |||
0339071 | |||
0339457 | |||
0339892 |
Customer inquiries related to this recall, as well as adverse reaction/events experienced with the product should be addressed to BD Customer Support at 1-844-8BD- LIFE (1-844-823-5433); When calling Monday- Friday between the hours of 9 a.m. to 6 p.m. ET, say "Recall" when prompted. For additional information, customers can visit www.bd-chloraprep-action.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this drug product.
The U.S. Food and Drug Administration (FDA) has been notified of this recall.
FDA MedWatch Reporting
Adverse reactions/events experienced with the use of any of these products should also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form or submit by fax to 1-800-FDA-0178.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/ and Twitter @BDandCo.
Contacts: | |
Media: | Investors: |
Troy Kirkpatrick | Kristen M. Stewart, CFA |
VP, Public Relations | SVP, Strategy & Investor Relations |
858.617.2361 | 201.847.5378 |
____________________
1 https://www.bd.com/documents/labels/IP_ChloraPrep-26mL-Clear_PL.pdf
SOURCE BD (Becton, Dickinson and Company)
These press releases may also interest you
|
News published on and distributed by: