Subject: SVY
GHIT Fund Announces New Investments: A Total of 1.37 Billion Yen in Drugs for Malaria, Chagas Disease, Leishmaniasis, Schistosomiasis, and Soil-Transmitted Helminths, and Diagnostics for Malaria, Buruli Ulcer, and Schistosomiasis
TOKYO, Sept. 28, 2020 /PRNewswire/ -- The Global Health Innovative Technology (GHIT) Fund announced today a total of 1.37 billion yen (US$13 million*) to invest in seven partnerships to develop new lifesaving drugs and diagnostics for malaria, Buruli ulcer, Chagas disease, leishmaniasis, schistosomiasis, and soil-transmitted helminths (STH). This includes three newly funded projects and four that will receive continued funding.** (Appendix 1 & 2)
"It is tremendously important that research and development (R&D) for neglected diseases, temporarily paused during the spring and summer due to the pandemic, are gradually resuming all over the world," said GHIT's CEO and Executive Director, Catherine Ohura.
"In the midst of the COVID-19 crisis and regardless of this pause, product development for malaria, tuberculosis, and neglected tropical diseases has remained our crucial priority. We continue to work with our domestic and overseas product development partners with the same strong sense of urgency. We are proud of our partners' resilience and unwavering commitment to developing new tools for neglected patients," she added.
"We are very thrilled about the new investments in clinical stages, such as the development of a treatment for leishmaniasis, entering Phase I trials in humans, and the development of a rapid diagnostic kit for schistosomiasis, using patient specimens in the Philippines and Kenya. Additionally, other investments in the target and discovery stage include such innovative approaches as a recombinant anthelmintic protein drug for STH, rapid diagnostic technology for Buruli ulcer, and compound screening in collaboration with multiple Japanese companies. These new candidates will strengthen our portfolio and provide new hope for patients and healthcare professionals," Ohura continued.
As of September 29, GHIT's portfolio includes 50 ongoing projects: 26 discovery projects, 16 preclinical projects and eight clinical trials (Appendix 3). The total amount of investments since 2013 is 22.3 billion yen (US$211 million).
* USD1 = JPY105.36, the approximate exchange rate on August 31, 2020.
** These awarded projects were selected from a number of proposals to the RFP2020-001 for Target Research Platform, Screening Platform, Hit-to-Lead Platform, and Product Development Platform, which was open for applications from November 2019 to March 2020. The GHIT board conducted in June 2020 approved these new investments.
The GHIT Fund is a Japan-based international public-private partnership fund (PPP) between the Government of Japan, multiple pharmaceutical companies, the Bill & Melinda Gates Foundation, the Wellcome Trust, and the United Nations Development Programme (UNDP). The GHIT Fund invests and manages an R&D portfolio of development partnerships aimed at neglected diseases, such as malaria, tuberculosis and neglected tropical diseases that afflict the world's vulnerable and underserved populations. The GHIT Fund mobilizes the Japanese industry, academia, and research institutes to create new drugs, vaccines, and diagnostics for malaria, tuberculosis, and neglected tropical diseases, in collaboration with global partners.
Appendix.1 New Investments | |||||
ID/Status | Project Title | Collaboration Partners | Disease/ | Stage | Awarded Amount |
G2020-104 New project | A schistosomiasis rapid diagnostic test to support control programmes in monitoring treatment impact and reassessment mapping | Institute of Tropical Medicine, Nagasaki University (NUITM), Foundation for Innovative New Diagnostics (FIND), Leiden University Medical Center (LUMC), Department of Parasitology, Merck KGaA | Schistosomiasis Diagnostics | Product Development | ¥373,397,356 (US$3,544,014) |
G2020-108 Continued project | Clinical development of CpG-D35 for combined treatment of cutaneous leishmaniasis | The University of Tokyo, Ajinomoto Bio-Pharma Services, GeneDesign (GeneDesign), Drugs for Neglected Diseases initiative (DNDi) | Leishmaniasis Drug | Phase 1 Clinical Development | ¥692,399,748 (US$6,571,752) |
T2020-162 Continued project | Cry5B optimization for Trichuris - whipworm | Kao Corporation, PATH, University of Massachusetts Medical School | Soil-transmitted helminths Drug | Lead Optimization | ¥92,302,072 (US$876,064) |
S2020-112 New project | Screening project between Daiichi-Sankyo, MMV, Eisai and Takeda | Millennium Pharmaceuticals, Inc. (Takeda Pharmaceutical Company Limited), Eisai Co., Ltd., Daiichi Sankyo Company Limited, Medicines for Malaria Venture (MMV) | Malaria Drug | Hit Identification | ¥21,093,600 (US$200,205) |
S2020-121 Continued project | Screening project between Daiichi Sankyo RD Novare and DNDi | Daiichi Sankyo RD Novare Co.,Ltd, Drugs for Neglected Diseases initiative (DNDi) | Chagas disease, Leishmaniasis Drug | Hit Identification | ¥8,000,000 (US$75,930) |
T2020-153 Continued project | Towards the rapid diagnosis of malaria hypnozoite infection: feasibility studies | Institute of Tropical Medicine, Nagasaki University (NU-ITM), National Institute of Technology, Kumamoto College (NIT-KC), Biomedical Primate Research Centre (BPRC) | Malaria Diagnostics | Technical Feasibility | ¥99,980,967 (US$948,946) |
T2020-161 New project | Development of 'all-in-one' diagnostic kit for Buruli ulcer using lateral flow DNA-chromatography | Teikyo University, Keio University School of Medicine, Tohoku Bio-Array (TBA) Co., Ltd, Nagasaki University, FASMAC CO., LTD, Raoul Follereau Institute Côte d'Ivoire, Pasteur Institute Côte d'Ivoire, Hope Commission International | Buruli ulcer Diagnostics | Concept Development | ¥89,558,400 (US$850,023) |
*All amounts are listed at the exchange rate of USD1 = JPY105.36, the approximate exchange rate on August 31, 2020. | |||||
Appendix.2 Project Details | |
G2020-104 | |
Project Title | A schistosomiasis rapid diagnostic test to support control programmes in monitoring treatment impact and reassessment mapping |
Collaboration Partners | Institute of Tropical Medicine, Nagasaki University (NUITM), Foundation for Innovative New Diagnostics (FIND), Leiden University Medical Center (LUMC), Department of Parasitology, Merck KGaA |
Disease | Schistosomiasis |
Intervention | Diagnostics |
Stage | Product Development |
Awarded Amount | ¥373,397,356 (US$3,544,014) |
Status | New project |
Summary | Schistosomiasis (SCH) is a major neglected tropical disease impacting the health of the lowest income populations. More than 220 million people are affected by this disease, with ~90% of the burden found in sub-Saharan Africa. Current WHO guidelines for the diagnosis of SCH recommend examination of stool and/or urine samples by microscopy, for the presence of schistosome eggs. Whilst these tests are useful in settings with moderate to high intensity infections, they become futile in settings where prevalence and intensity are low, due to their poor sensitivity. To account for this, sampling is repeated on multiple days and several slides are examined by trained microscopists, rendering the methods time-consuming and challenging to deploy. To address this, the Foundation for Innovative New Diagnostics of Geneva, Switzerland (FIND), together with Leiden University Medical Center of Leiden, Netherlands (LUMC), Merck KGaA of Darmstadt, Germany and Nagasaki University Institute of Tropical Medicine of Nagasaki, Japan (NUITIM) are developing an easy-to-use, accurate and affordable SCH rapid diagnostic test (RDT) with a sensitivity comparable to repeated microscopy that detects circulating anodic antigen (CAA), an antigen that is secreted continuously by living schistosomes. The results of the test will be obtained within 20 minutes. The RDT is being developed and optimized by Mologic Ltd under a sub-contract to FIND, including incorporation of engineered antibodies, novel detection nanoparticles and materials to enable detection of CAA in finger-stick whole-blood. The goal of this project is to develop and carry out clinical validation of an RDT that is simple to use, instrument free and affordable, to support SCH control programmes in monitoring the impact of treatment as well as for re-assessment mapping, that is effective on all schistosome species that are of public health importance. The partners in this project will (a) conduct field evaluations on semi-quantitative prototype SCH RDT, (b) optimize the RDT, design-lock and transfer to manufacturing, (c) validate the performance of the RDT in the detection of the major schistosome species, and determine its suitability as a replacement of current microscopy-based diagnostic tests, and (d) develop an access strategy for the SCH RDT. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/167/en |
G2020-108 | |
Project Title | Clinical development of CpG-D35 for combined treatment of cutaneous leishmaniasis |
Collaboration Partners | The University of Tokyo, Ajinomoto Bio-Pharma Services, GeneDesign (GeneDesign), Drugs for Neglected Diseases initiative (DNDi) |
Disease | Leishmaniasis |
Intervention | Drug |
Stage | Phase I Clinical Trial |
Awarded Amount | ¥692,399,748 (US$6,571,752) |
Status | Continued project |
Summary | Cutaneous leishmaniasis (CL) is a severely neglected tropical disease. It is endemic in 87 countries worldwide, mainly affecting poor populations in developing countries. The World Health Organization (WHO) has estimated that there are around 0.6 to 1.2 million new CL cases every year. While CL is not life-threatening, it is a disfiguring disease that results in stigma and economic loss. Currently, there are no satisfactory treatments for any form of CL. Treatments recommended for CL have sub-optimal effectiveness and have long depended on antiquated drugs. CpG-D35 is being developed as a combination therapy for the treatment of patients with CL. It triggers the Toll-like receptor-9 (TLR-9) expressed on plasmacytoid dendritic cells and thereby activates the innate and adaptive immune system of the host. Data generated to date support the hypothesis that CpG-D35 alone or in combination with chemotherapy will reduce infection and accelerate the healing of CL lesions, as demonstrated in preclinical studies with CpG-D35. Consequently, CpG-D35 is expected to improve CL patient care. This is a continuation from the project previously funded by the GHIT Fund, aiming to: 1. Determine the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) after single subcutaneous dose of CpG-D35 in heathy volunteers (HVs), compared to matching placebo (SAD study) and after multiple doses administration in subjects having active infection with L. major parasites (MAD study). 2. Refine current active pharmaceutical ingredient (API) manufacturing process to improve efficiency/overall quality of CpG-D35 drug substance. 3. Develop a more concentrated, affordable, and field-adapted subcutaneous dosage form for later-stage clinical trials. 4. Further optimize the CAL-1 potency assay used for CpG-D35 quality control and stability testing. A single ascending dose (SAD) study will be conducted in the UK. A randomized, double-blind, placebo-controlled, single-centre study with CpG-D35 administered subcutaneously to healthy subjects will aim to assess safety and tolerability of CpG-D35 after a single dose compared to a matching placebo. As secondary objectives, PK and PD of CpG-D35 after single dose will be investigated, including but not limited to changes in cytokine levels in plasma over time as PD markers. If no safety concerns are encountered during the SAD study, then a Phase Ib, multiple ascending dose (MAD) study will be implemented. It will be a randomized, double-blind, placebo-controlled, multiple-dose escalation study to investigate safety, tolerability, and immunogenicity of CpG-D35 in patients with CL lesions due to L. major. The study will be performed in a single-site hospital specialized in Phase Ib studies in Turkey. Secondary objectives of this study are: 1) Assess PK and PD of CpG-D35; 2) Characterize the immune response produced by CpG-D35 in L. major infected patients; 3) Determine the value of using CXCL10 as PD marker; and 4) Establish CL lesions' time to heal after multiple doses of CpG-D35. Refinement of the current API manufacturing process will include optimization of the coupling step and washing step(s), change to other sulfurizing reagents, and preparation of a demonstration batch. To assess the feasibility of producing a more concentrated liquid formulation, the ease with which current liquid clinical formulation can be reconstituted after lyophilisation with lower volumes will also be evaluated. Different excipients will be screened in parallel to provide solutions that can be reconstituted to tonicity. The in vitro CAL-1 cell-based potency assay will also be refined. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/168/en |
T2020-162 | |
Project Title | Cry5B optimization for Trichuris - whipworm |
Collaboration Partners | Kao Corporation, PATH, University of Massachusetts Medical School |
Disease | Soil-transmitted helminths |
Intervention | Drug |
Stage | Lead Optimization |
Awarded Amount | ¥92,302,072 (US$876,064) |
Status | Continued project |
Summary | Soil-transmitted helminth (STH) infections are a significant neglected tropical disease affecting approximately 1.5 billion people worldwide, or 24% of the world's population. The different species of intestinal parasitic worms (hookworm, roundworm, and whipworm) infecting humans are transmitted through contaminated soil, placing the heaviest burden on rural, low socioeconomic status communities lacking access to clean water and sanitation. Infected infants and children are at highest risk of mortality and morbidity from STH infections. The insidious effects on health and quality of life resulting from STH infections include low birth weight, iron deficiency anemia, chronic malnutrition, stunting, impaired growth and physical development, cognitive development disorders, delayed educational advancement, and a negative impact on economic development. In 2018, over 676 million school children were treated with anthelminthic medicines in endemic countries, but this only accounts for 53% of all children at risk. This project seeks to further the development of a new first-in-class broad-spectrum anthelmintic drug product that can serve as an inexpensive and potent alternative or complement to benzimidazole (e.g., albendazole and mebendazole) treatments currently used to combat STH infections and associated disease burden. Building on the work from the previous grant, the objective of this project is to optimize the Bacillus thuringiensis crystal (Cry) protein Cry5B lead sequence for Trichuris, commonly known as whipworm, which is the most difficult target because of its feeding habits and its location in the large intestine. Trichuris trichiura is a parasitic nematode that infects humans to cause trichuriasis, also known as human whipworm infection. Whipworms are fully sensitive to Cry5B and Cry5B variants tested in vitro. Based on previous studies, naturally occurring amino acid substitutions may significantly increase the bioactivity and effectiveness of Cry5B protein variants against hookworms. Variant screening will improve two parameters of Cry5B protein activity: effective dose and time to kill. These studies will result in the identification of a Cry5B variant lead candidate that will bring the whipworm effective dose down to the hookworm dose of 1 mg/kg. In exploratory studies conducted under the previous grant, the University of Massachusetts Medical School (UMMS) team began testing the activities of several Cry proteins and natural Cry5B amino acid variants against Trichuris. The sequence differences between Cry5B and variant Cry5B and other Trichuris-active toxins are hypothesized to affect toxin stability, activation, or receptor avidity. Under this project, UMMS will systematically test Cry variants for improved activity against Trichuris and, secondarily, hookworm (roundworm activity tracks with hookworm activity). Identified improved Cry5B amino acid variants and new Cry proteins will be produced by the Kao Corporation as Inactivated Bacterium with Cytosolic Crystal (IBaCC) in their Bacillus subtilis-based expression system, and then tested in vitro and in vivo at UMMS. The robust Bacillus subtilis expression technology for producing large amounts of recombinant Cry5B anthelminthic protein achieves a level expression of several grams per liter of culture, with the potential for further improvement. Our technology will produce and purify Cry5B at the scale necessary for biochemical characterization, purification, and subsequent in vitro and in vivo bioactivity testing to select the lead potency Cry5B protein variant candidates for advancement. As protein variants superior to canonical Cry5B are identified, they will be tested in vivo against whipworm infections in mice. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/174/en |
S2020-112 | |
Project Title | Screening project between Daiichi-Sankyo, MMV, Eisai and Takeda |
Collaboration Partners | Millennium Pharmaceuticals, Inc. (Takeda Pharmaceutical Company Limited), Eisai Co., Ltd., Daiichi Sankyo Company Limited, Medicines for Malaria Venture (MMV) |
Disease | Malaria |
Intervention | Drug |
Stage | Hit Identification |
Awarded Amount | ¥21,093,600 (US$200,205) |
Status | New project |
Summary | This is a screening project between Daiichi-Sankyo, MMV, Eisai and Takeda |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/176/en |
S2020-121 | |
Project Title | Screening project between Daiichi Sankyo RD Novare and DNDi |
Collaboration Partners | Daiichi Sankyo RD Novare Co.,Ltd, Drugs for Neglected Diseases initiative (DNDi) |
Disease | Chagas disease, Leishmaniasis |
Intervention | Drug |
Stage | Hit Identification |
Awarded Amount | ¥8,000,000 (US$75,930) |
Status | Continued project |
Summary | This is a screening project between Daiichi Sankyo RD Novare and DNDi. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/177/en |
T2020-153 | |
Project Title | Towards the rapid diagnosis of malaria hypnozoite infection: feasibility studies |
Collaboration Partners | Institute of Tropical Medicine, Nagasaki University (NU-ITM), National Institute of Technology, Kumamoto College (NIT-KC), Biomedical Primate Research Centre (BPRC) |
Disease | Malaria |
Intervention | Diagnostics |
Stage | Technical Feasibility |
Awarded Amount | ¥99,980,967 (US$948,946) |
Status | Continued project |
Summary | While clinical malaria cases in the Asia-Pacific and the Americas have gone down >90% in the last decade, a shift in malaria species composition has been observed, with Plasmodium vivax now being the predominant species outside Africa. This shift may relate to the unique biology of P. vivax, including the relapsing phenotype from dormant liver stages (hypnozoites). In the light of the United Nations Sustainable Development Goals (By 2030, end the epidemics of AIDS, tuberculosis, malaria...") and in the era of pursuing malaria eradication, an effective strategy to handle P. vivax malaria is indispensable. Asymptomatic hypnozoite infections form a hidden parasite reservoir in the human population that can give rise to new symptomatic and transmissible malaria weeks, months or years after primary infection, without new infection through mosquito bites. Proper diagnostic tools to identify hypnozoite-infected individuals are currently lacking, and this is mentioned as a challenge in "WHO Global Technical Strategy for Malaria 2016?2030". Identification of putative metabolite markers for malaria hypnozoite infection. Under our previous RFP (T2017-105) we pioneered an in vitro Proof-of-Concept (PoC) towards identifying targets for diagnostic tools for malaria hypnozoites, exploiting our unique experience in in vitro P. cynomolgi hypnozoite cultures (an accessible proxy to P. vivax with near identical biology), as well as in sensitive metabolomics. Specific metabolites have been identified and prioritized based on the unique signatures found in hypnozoite-enriched cultures. A second-phase in vivo feasibility study using the P. cynomolgi-rhesus monkey model is now warranted to determine whether the specific signatures detected in the in vitro PoC are confirmed in vivo and can thus be pursued in the subsequent development phase of a rapid diagnostic test for hypnozoite infection. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/170/en |
T2020-161 | |
Project Title | Development of 'all-in-one' diagnostic kit for Buruli ulcer using lateral flow DNA-chromatography |
Collaboration Partners | Teikyo University, Keio University School of Medicine, Tohoku Bio-Array (TBA) Co., Ltd, Nagasaki University, FASMAC CO., LTD, Raoul Follereau Institute Côte d'Ivoire, Pasteur Institute Côte d'Ivoire, Hope Commission International |
Disease | Buruli ulcer |
Intervention | Diagnostics |
Stage | Concept Development |
Awarded Amount | ¥89,558,400 (US$850,023) |
Status | New project |
Summary | Buruli ulcer is a cutaneous mycobacterial infection, which is reported in about 2,000 new patients annually worldwide, mainly in West Africa. Children are mainly affected due to infection with the acid-fast bacteria Mycobacterium ulcerans (M. ulcerans) that exists in the water system of the environment such as ponds and rivers. When diagnosis or treatment is delayed, a wide range of intractable ulcers will form on the skin, leaving aftereffects such as limited joint movement after healing. It is often difficult to distinguish Buruli ulcers from other skin ulcers of various causes, and the only way to make a reliable diagnosis is to take a sample from the ulcer and confirm it by PCR. However, it is difficult to perform such a test that requires such advanced technology and expensive equipment in the West Africa where the number of patients is high. We have established a method for amplifying M. ulcerans DNA without using expensive equipment and a determination method by DNA chromatography. We will combine these techniques to develop a new diagnostic method for Buruli ulcer. As one of the skin NTDs, there is no point-of-care diagnostic method for Buruli ulcer, which results in delay in diagnosis and treatment and many patients have to live with serious sequelae. The number of new cases of Buruli ulcer reported to the WHO is about 2,000 per year, which is due to the fact that the diagnosis itself has not been made because the disease itself is not well recognized. This indicates that there are many cases in which the patient is left without being properly diagnosed due to lack of proper diagnostic method, and wrong treatment might be given. In order to perform PCR, which is currently the only diagnostic method recommended by WHO, a specialized laboratory with expensive equipment and technologists with a high level of knowledge and skills are required. Therefore, it is not practical to perform PCR in endemic area of Buruli ulcer. In this project, we will develop a diagnostic kit for Buruli ulcer that can be tested by anyone in anywhere. In order to perform DNA isothermal amplification methods such as the LAMP method, it is necessary to mix special enzymes and various reagents with the sample in the correct proportions. In this project, we will develop a small kit in which these reagents are fixed and dried in a stabilized state, and the reaction takes place simply by adding a sample. By adding the solution after the DNA amplification reaction is completed, the amplified DNA will be developed by DNA chromatography, and if M. ulcerans DNA is present, a visually recognizable line will appear. The detection sensitivity is about the same as the PCR method, and we aim to develop a kit that greatly reduces the cost and time required for testing. The kit developed in Japan will be used by local medical staff in Côte d'Ivoire, further improved to produce the final kit. |
Project Detail | https://www.ghitfund.org/investment/portfoliodetail/detail/173/en |
*All amounts are listed at the exchange rate of USD1 = JPY105.36, the approximate exchange rate on August 31, 2020. | |
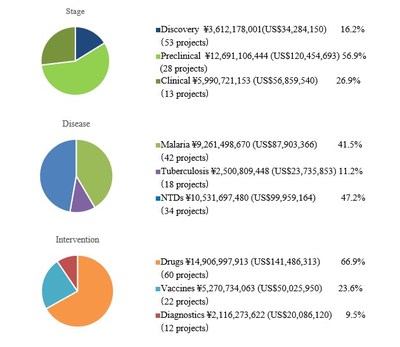
Appendix.3 Investment Overview (As of September 29, 2020)
1. Investment to date
Total investments 22.3 billion yen (US$211 million*)
Total invested Projects 94?active projects 50, completed projects 44?
2. Portfolio analysis (active projects + completed projects)
*All amounts are listed at the exchange rate of USD1 = JPY105.36, the approximate exchange rate on August 31, 2020.
To know more about GHIT investments, please visit
Investment Overview: https://www.ghitfund.org/investment/overview/en
Portfolio: https://www.ghitfund.org/investment/portfolio/en
Advancing Portfolio: https://www.ghitfund.org/investment/advancingportfolio/en
Clinical Candidates: https://www.ghitfund.org/investment/clinicalcandidates/en
*All amounts are listed at the exchange rate of USD1 = JPY105.36, the approximate exchange rate on August 31, 2020.
SOURCE GHIT Fund
These press releases may also interest you
|
News published on and distributed by: