Subject: AVO
FDA Issues Mercury Amalgam Filling Warning, Group Calls For Even More Protection
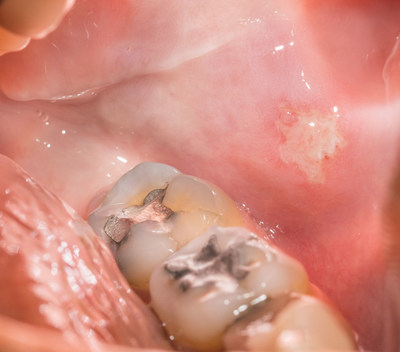
CHAMPIONSGATE, Fla., Sept. 25, 2020 /PRNewswire/ -- The International Academy of Oral Medicine and Toxicology (IAOMT) is commending the Food and Drug Administration (FDA) for its statement yesterday that warns high-risk groups about the potential for adverse health outcomes from dental amalgam mercury fillings. However, the IAOMT, who has demanded more stringent protection from dental mercury for more than three decades, is now calling on the FDA for even more protection for all dental patients.
Yesterday, the FDA updated its recommendations concerning dental amalgam fillings and cautioned that "harmful health effects of mercury vapor released from the device" could impact high-risk populations. Susceptible groups advised to avoid getting mercury amalgam fillings include pregnant women and fetuses; women planning to become pregnant; nursing women and their newborns and infants; children; people with neurological disease such as multiple sclerosis, Alzheimer's disease or Parkinson's disease; people with impaired kidney function; and people with known heightened sensitivity (allergy) to mercury or other components of dental amalgam.
"This is certainly a step in the right direction," Jack Kall, DMD, IAOMT Executive Chairperson of the Board stated. "But mercury shouldn't be placed in anyone's mouth. All dental patients need to be protected, and dentists and their staff also need to be protected from working with this toxic substance."
Dr. Kall is among a number of IAOMT member dentists and researchers who have testified to the FDA about the dangers of dental amalgam over the course of several decades. When the IAOMT was founded in 1984, the non-profit vowed to examine the safety of dental products by relying upon peer-reviewed scientific research. In 1985, after mercury vapor release from fillings was established in the scientific literature, the IAOMT issued a declaration that the placement of silver/mercury dental amalgam fillings should cease until evidence of safety could be produced. No evidence of safety was ever produced, and meanwhile, the IAOMT has collected thousands of peer-reviewed scientific research articles to support their position that dental mercury usage should end.
"Because of our advocacy for safer, evidence-based dentistry, we have finally convinced the FDA that, at a minimum, some people are at risk," David Kennedy, DDS, IAOMT Board of Directors, asserted. "Over 45% of dentists worldwide are still estimated to be using amalgam, including a large majority of dentists for military and welfare agencies. It should not have taken 35 years to get to this point, and FDA now needs to protect everyone."
The IAOMT has compared the delayed route in safety regulations for mercury fillings to the same scenario that occurred with cigarettes and lead-based products such as gasoline and paint. The organization is also concerned about increased mercury exposure to patients and dental professionals when amalgam fillings are removed unsafely, as well as the health risks caused by fluoride exposure.
Contact: | David Kennedy, DDS, IAOMT Public Relations Chair, [email protected] |
International Academy of Oral Medicine and Toxicology (IAOMT) | |
Phone: (863) 420-6373 ext. 804; Website: www.iaomt.org |
SOURCE International Academy of Oral Medicine and Toxicology
These press releases may also interest you
|
News published on and distributed by: