Subject: IMA
ReddyPort receives CE Mark Approval for ReddyPorttm Elbow Non-Invasive Ventilation product
SALT LAKE CITY, June 2, 2020 /PRNewswire/ -- ReddyPort, a medical technology company focused on solutions that enable better care experiences for patients on non-invasive ventilation (NIV), announced today that it has received Conformité Européene (CE) Mark approval for ReddyPorttm Elbow. The CE marking confirms that ReddyPort, a suite of solutions that promotes NIV success, and allows clinicians to perform procedures through the ventilation mask without interrupting therapeutic pressure meets the requirements of the European Medical Devices Directive (MDD). This now allows ReddyPort to commercialize ReddyPorttm Elbow across the European Union and other CE Mark geographies.
"CE Mark is an important milestone and validation of our vision to make ReddyPort the standard of care for patients on NIV," said Scott Bostick, chief executive officer of ReddyPort. CE Mark approval means we can help patients in Europe who struggle tolerating NIV therapy due to known issues while wearing the mask; dry-mouth, phlegm build-up, and difficulty communicating. ReddyPorttm Elbow and oral care products help improve tolerance, patient compliance, and comfort, leading to more successful NIV. Supporting a successful NIV is especially important now as clinicians need it most during the COVID-19 pandemic."
NIV is the first line of therapy in respiratory insufficiency or failure, commonly seen with COPD, CHF, asthma, pneumonia or ARDS1. NIV intolerance can lead to failure and escalation of care with mechanical ventilation. ReddyPort products promote NIV success, by empowering clinicians and patients with simplified oral access without interrupting patient therapy.
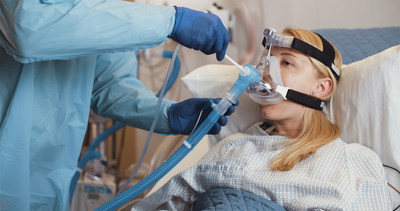
ReddyPort features a proprietary elbow with self-sealing valve that provides access to the patient's mouth. ReddyPorttm Elbow, in combination with ReddyPorttm NIV Maintenance Kit, allows the ability to clean and moisturize the patient's mouth without mask removal. Oral hygiene is proven to help reduce risk of infection and improve patient satisfaction.
Reference
1 Rochwerg B, Brochard L, Elliott MW, Hess D, et al. Official ERS/ATS Clinical Practice Guidelines: Noninvasive Ventilation for Acute Respiratory Failure. Eur Respir J. 2017: 31;50(2):1602426.
About ReddyPort
ReddyPort is focused on enabling a better care experience for patients on NIV, providing solutions to help improve satisfaction, reduce risk and cost, enable better workflows, and enhance quality of care.
For more information, call us at 801.899.3036, visit www.reddyport.com or connect with us on Linkedln.
SOURCE ReddyPort
These press releases may also interest you
|
News published on and distributed by: