Subjects: PDT, TRI
UK Biotech Imophoron Uses New Vaccine Platform to Develop COVID-19 Candidates
BRISTOL, England, April 7, 2020 /PRNewswire/ -- UK biotech Imophoron has produced multiple COVID-19 vaccine candidates, based on its novel vaccine platform (named the ADDomer©), within weeks of the virus sequence being made available.
- Pre-clinical trials to begin within weeks
- The UK start-up is looking for partners to further the development of the COVID-19 candidates and the ADDomer platform
- Vaccines based on the ADDomer platform are likely to require no adjuvant, no refrigeration and have reduced risk of side effects
Imophoron has joined the University of Bristol's COVID-19 Emergency Research (UNCOVER) Group, and is leading on vaccine development. Together, they will soon test their COVID-19 candidate vaccines in-vivo in a pre-clinical programme.
Frederic Garzoni, Founder and CEO at Imophoron, said: "We have optimized our process and can now design and roll-out potential vaccines in about two weeks, ready for testing. With our technology, we hope to contribute to resolving the major health and economic threats caused by emerging viruses such as COVID-19."
The start-up, based at the Unit DX Incubator in Bristol, UK, is developing a new, highly adaptable, easy-to-manufacture, rapid-response platform for vaccines to combat present and future infectious diseases. A key benefit is the speed with which candidate vaccines can be identified and could be manufactured in large quantities. Imophoron's vaccines are extremely stable and require no refrigeration, potentially enabling unrestricted distribution world-wide. Importantly the high specificity of the vaccine particle promises a reduced risk of potentially dangerous side effects, seen with some novel vaccines.
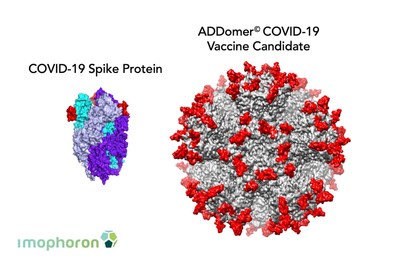
Professor Imre Berger, co-founder at Imophoron and Director of the University's Max Planck-Bristol Centre for Minimal Biology, added: "COVID-19 (SARS-CoV-2) infects cells using its so-called `Spike' protein. Most COVID-19 vaccines now being fast-tracked present the complete Spike to the immune system, which reacts by making antibodies. This approach risks inducing antibodies that bind to the wrong parts of the Spike and could make the disease even worse. In vaccines for SARS-CoV-1, this sometimes resulted in severe lung tissue damage; Imophoron's vaccines, in contrast, present only very specific parts of the Spike essential for cell entry and are potentially much less prone to this risk."
Imophoron's platform, the ADDomer©, is a synthetic, self-assembling, nature-inspired virus-like particle (VLP). Currently at pre-clinical stage, vaccines based on the ADDomer© will need to be studied in human clinical trials once they have completed pre-clinical tests.
Professor Adam Finn, Director of the Bristol Children's Vaccine Centre at Bristol Medical School and co-ordinator of UNCOVER, explained: "We believe the approach has a number of potential advantages, including avoidance of induction of disease-enhancing antibody responses, ready manufacture and thermostability, avoiding the need for cold chain storage. The proven track record of the VLP concept for presentation of antigens to the human immune system, inducing substantial long-lasting antibody responses and clinical protection, also favours this approach."
Imophoron was founded in December 2017 by Frederic Garzoni and Imre Berger.
The journal Science Advances published a paper unveiling the technology in September 2019.
Photo - https://mma.prnewswire.com/media/1142077/Imophoron_Unit_DX_Vaccine_Candidate_Image.jpg
Adam Finnimore, [email protected]
These press releases may also interest you
|
News published on and distributed by: