Subjects: WOM, MAT
Top Gynecologist Issues Warning on Small Wearable Vibrators
MESA, Ariz., Jan. 21, 2020 /PRNewswire/ -- The Marchand Institute for Minimally Invasive Surgery released a warning today regarding new lines of small adult toys that are marketed as being as wearable as fashion jewelry and doubling as a functioning vibrating adult toy. These new devices are detachable from the necklace chain, and once detached they have an extremely thin diameter. As the devices are designed to stimulate the clitoral area, this raises a serious concern for the devices entering, and becoming lost within the urethra.
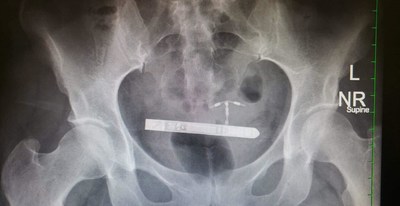
Surgeons at The Marchand Institute for Minimally Invasive Surgery have removed the device from a patient's bladder already using a cystoscopic approach.
"Women need to know about the dangers of these devices," said Dr. Greg J. Marchand, director of the Institute. "The diameter of these devices is only approximately 1.2cm, or about the same as a 36 french catheter. If the device inadvertently slips into the urethra, it can easily be lost and will not be recoverable without surgery."
The devices do come with a brief warning label stating that the devices are not for "Vaginal, Anal or Urethral Use." The Institute said that this warning was "ridiculously insufficient," as the risk for losing the device in the bladder was very much a also danger for users attempting clitoral stimulation, and the risk was not at all limited to users intentionally entering the urethra.
Making matters worse, health professionals do not routinely search the bladder for foreign bodies, as it is unusual for a large object to enter the bladder because of the narrow opening of the urethra. Therefore the device could be difficult to locate by radiology or surgical exploration. It is much more common for an object to penetrate the posterior wall of the vagina and enter the abdominal cavity, or to be unretrievable in the rectum. Therefore location and removal of these devices can be difficult and could lead to unnecessary extra surgery or complications.
The Institute recommends that all women avoid rod shaped vibrators with an extremely narrow diameter, and instead choose devices with a wider diameter, at least as thick as a marker or thumb.
For more information, arrange interviews or for additional media please contact Maria at [email protected].
Maria Sainz
Public Relations Manager
The Marchand Institute for Minimally Invasive Surgery
480-695-5211
[email protected]
This release was issued through WebWire (R). For more information, visit http://www.webwire.com.
SOURCE The Marchand Institute for Minimally Invasive Surgery
These press releases may also interest you
|
News published on and distributed by:



