Subject: TRI
Patient implanted in EndoStim's LESS GERD clinical trial for gastroesophageal reflux disease (GERD)
DALLAS, June 15, 2017 /PRNewswire/ -- EndoStim, Inc., announced that the first patient at the Stanford University School of Medicine has been implanted with the EndoStim device as part of the multicenter Lower Esophageal Sphincter Stimulation for GERD (LESS GERD) trial. The EndoStim system is a minimally-invasive implantable device designed to provide long-term reflux control by restoring normal function to the esophagus through neurostimulation. Stanford is one of 16 sites worldwide participating in the clinical trial.
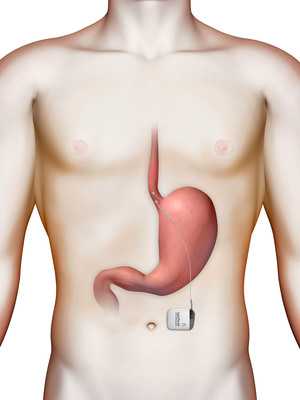
GERD affects nearly 65 million people in the United States[1]. It occurs when stomach acid or other stomach contents flow back into the esophagus, often caused by a weak valve, or sphincter, between the esophagus and the stomach called the lower esophageal sphincter (LES). Frequent and troublesome symptoms can include heartburn, regurgitation, sleep disruption, vocal impairment and respiratory complications. Most GERD is successfully treated with acid blocking medications such as proton pump inhibitors (PPI). However, nearly 30 percent of patients on PPI medication continue to suffer from symptoms. The traditional anti-reflux surgery is laparoscopic fundoplication surgery, a procedure in which the surgeon wraps the top of the stomach around the lower esophagus to reinforce the lower esophageal sphincter. While typically effective, fundoplication can cause significant side effects.
The LESS GERD trial will evaluate the safety and efficacy of the EndoStim Lower Esophageal Sphincter (LES) Stimulation System in patients with gastro-esophageal reflux disease (GERD) who experience symptoms despite taking high-dose proton pump inhibitor (PPI) medications.
"We are excited to start the LESS GERD Clinical trial to generate evidence for a novel new treatment for Chronic GERD, an undertreated disease that is disrupting the lives of millions of patients worldwide," said Rohan Hoare, Ph.D., President and Chief Executive Officer of EndoStim. "Unlike many common treatment options that work to alleviate GERD symptoms, EndoStim targets the underlying pathophysiology of GERD with the potential to restore normal function to the lower esophageal sphincter (LES)."
About the Lower Esophageal Sphincter Stimulation for GERD (LESS GERD) Trial
The LESS GERD trial will examine the effects of the EndoStim LES Stimulation System on GERD outcomes such as: esophageal acid exposure; GERD symptoms (heartburn and regurgitation); ability to avoid dependence on PPI medications; and the effect on overall quality of life. A minimum of 110 subjects will be implanted with the EndoStim device. The study is open to GERD patients who are between the ages of 22 and 75; have been diagnosed with GERD; have taken daily PPI medication and whose GERD symptoms are not completely resolved or have side effects from the PPI; and have had no prior surgery involving the esophagus.
Patients seeking information on the trial should visit: www.lessgerd.com.
About EndoStim
EndoStim is a medical device company based in Dallas, Texas, and Nijmegen, The Netherlands, developing and commercializing a revolutionary treatment for GERD. The EndoStim LES Stimulation System is CE Marked for patients with gastroesophageal reflux disease with symptom duration of six months or longer, and is available in a number of countries throughout Europe, Latin America, and Asia Pacific. The EndoStim system is not approved for sale in the US and is limited by US federal law to investigational use only. For more information, visit www.endostim.com.
[1] National Institute of Diabetes and Digestive and Kidney Diseases, Definition and Facts for GER and GERD, http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/ger-and-gerd-in-adults/Pages/definition-facts.aspx. Accessed June 9, 2016.


SOURCE EndoStim, Inc.
These press releases may also interest you
|
News published on and distributed by:



