Subjects: RCL, PSF
Public advisory - Unauthorized health products removed from sale from More to Shoppe Facebook store and seized from its Edson, Alberta, warehouse
OTTAWA, ON, Jan. 20, 2023 /CNW/ -
Summary
- Product: Various unauthorized health products labelled to contain prescription drugs
- Issue: Health products ? Product safety
- What to do: Do not use these products. Return them to your local pharmacy for proper disposal. Consult a health care professional if you have used any of these products and have health concerns. Prescription drugs can only be legally sold with a prescription. Buy your prescription drugs from licensed pharmacies only.
Images
Affected products
Product & Promoted Use | Prescription drug on the label |
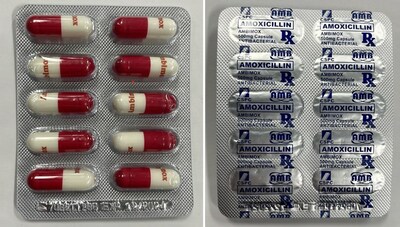
Ambimox Antibiotic | Labelled to contain amoxicillin |
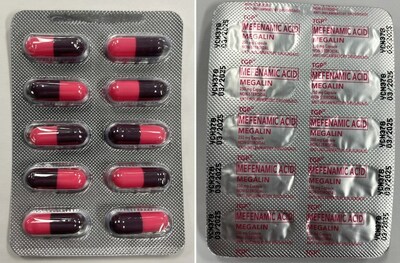
Megalin Pain relief | Labelled to contain mefenamic acid |
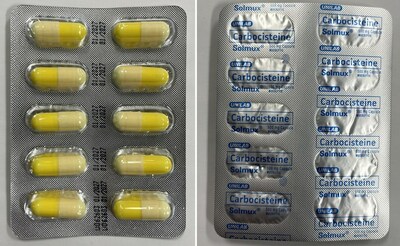
Solmux Mucolytic (mucus thinner) | Labelled to contain carbocisteine |
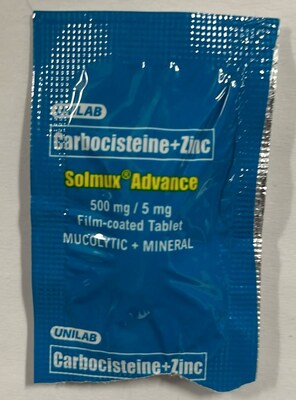
Solmux Advance Mucolytic (mucus thinner) | Labelled to contain carbocisteine |
Brilliant Skin Essentials Skin Lightening | Labelled to contain hydroquinone and tretinoin |
Brilliant Skin Essentials Skin Lightening | Labelled to contain hydroquinone and tretinoin |
Brilliant Skin Essentials Skin Lightening | Labelled to contain hydroquinone and tretinoin |
Health Canada removed various unauthorized health products from sale from More to Shoppe, an online Facebook store, and seized them from its warehouse in Edson, Alberta. The products are labelled to contain prescription drugs and may pose serious health risks.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, efficacy and quality and may pose a range of serious health risks. For example, they could contain high-risk ingredients, such as prescription drugs, additives or contaminants that may or may not be listed on the label. These ingredients could interact with other medications and foods. In addition, these products may not actually contain the active ingredients that consumers would expect them to contain to help maintain and improve their health.
Prescription drugs should only be used under the advice and supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects. Prescription drugs can only be legally sold with a prescription.
What you should do
- Do not use these products. Return the product to your local pharmacy for proper disposal.
- Consult a health care professional if you have used any of these products and have health concerns.
- Buy your prescription drugs only from licensed pharmacies.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related side effects or complaints to Health Canada.
Amoxicillin?is a prescription antibiotic drug used to treat certain bacterial infections. It should be used only under the supervision of a health care professional. Amoxicillin may cause severe allergic reactions with symptoms including swollen lips, face and throat, difficulty breathing, severe skin rashes and itchiness, nausea, vomiting, diarrhea, and signs of kidney and liver failure. It should not be used by patients with known allergies to drugs containing amoxicillin. Patients with allergies to other antibiotic types may also experience allergic reactions to amoxicillin. Misuse or overuse could lead to growth of bacteria that will not be killed by amoxicillin and means that the antibiotic may not work in the future when required (this is known as antibiotic resistance).
Carbocisteine is a prescription drug that is not approved for use in Canada. It is used in other countries to treat conditions associated with too much mucous in the respiratory tract. Side effects include diarrhea, nausea and heartburn. Serious allergic (e.g., anaphylactic) and skin reactions have been reported with its use. Carbocisteine can disrupt the lining of the stomach and can cause gastrointestinal bleeding in the elderly, those with a history of peptic ulcers, or patients taking medications known to cause gastrointestinal bleeds such as acetylsalicylic acid (ASA) or non-steroidal anti-inflammatory drugs (NSAIDs). The use of carbocisteine by pregnant people is not recommended.
Hydroquinone is a prescription drug when the product contains greater than a 2% concentration and a natural health product at a concentration of 2% or less. Prescription-strength hydroquinone should only be taken under the supervision of a health care professional. Hydroquinone is used topically (applied to the skin) to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding people, or for children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure.
Mefenamic acid, when taken orally (by mouth), is a prescription non-steroidal anti-inflammatory drug (NSAID). It is used to relieve muscle aches, headaches, dental pain and menstrual pain. It should only be taken under the supervision of a health care professional. This drug increases the risk of serious heart-related events (such as heart attack and stroke), high blood pressure, stomach or intestinal bleeding or ulcers, severe kidney or liver problems, and bleeding or clotting problems. Serious allergic reactions can occur, including skin rash and itching, that may also be in combination with chills, aches and other flu-like symptoms.
Tretinoin in topical format (applied to the skin) is a prescription drug used to treat acne. It should only be taken under the supervision of a health care professional. Topical tretinoin should not be used during pregnancy as it has been associated with birth defects. It should also not be used by those who are breastfeeding or by children under 12 years old, or by individuals who have inflamed or irritated skin, have a previous skin cancer or undiagnosed skin lesions, who are taking medicines that make their skin more sensitive to light, or who have an allergy to tretinoin. Tretinoin may cause pain, irritation, itchiness, redness, or swelling at the site of application. It may damage skin, change skin colour, and increase sensitivity to sunlight or tanning beds, causing sunburns. Using tretinoin in combination with hydroquinone may increase some of the side effects of tretinoin.
Également disponible en français
SOURCE Health Canada
These press releases may also interest you
|
News published on and distributed by: