Subjects: TRI, FDA
FDA Clears DyAnsys Neurostimulation Device First Relief to treat Diabetic Neuropathic Pain
PALO ALTO, Calif., July 14, 2022 /PRNewswire/ -- First Relief, a PENS (percutaneous electrical neurostimulation) device, has been cleared by the US Food and Drug Administration for multiple treatments up to 56 days for symptomatic relief of chronic, intractable pain from diabetic peripheral neuropathy, Dyansys Inc. has announced.
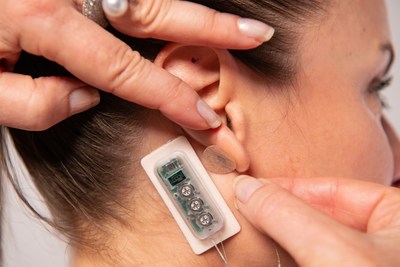
The wearable device placed on the ear administers continuous pulses of a low-level electrical current over several days.
"We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy, said DyAnsys CEO Srini Nageshwar. "First Relief offers a significant treatment option without drugs or narcotics."
The approval was based on a study that tested First Relief against a placebo and another device previously cleared by the US Food and Drug Administration.
The study was conducted at the Jeevak Multispeciality Hospital in Warangal, India, renowned for the treatment of diabetes. The single center, three-arm, randomized, controlled, parallel assignment, double blinded, prospective study involved 63 patients age 30 to 74.
The devices were applied on a bi-weekly basis for 16 weeks. The primary efficacy endpoint was pain intensity measured through Visual Analog Scale (VAS) score and the secondary efficacy endpoints are vibration perception threshold (VPT) value, insomnia severity index (ISI), overall neuropathy limitations scale (ONLS), Hamilton rating scale for anxiety.
The VAS pain score analysis showed a significant reduction in the pain score of patients being treated with First Relief from start of the treatment to the end. This improvement persisted throughout the 90-day follow-up, suggesting that the treatment was a long-term improvement in neuropathic pain and not a short-term improvement. The secondary outcome measures (VPT, Insomnia, ONLS and HAM) also showed similar improvements to the pain score, showing significant improvement in sleep and mood as the neuropathic pain decreased.
No complications or adverse events were observed in any of the subjects during the study period.
DyAnsys Inc. is a global company headquartered in California with subsidiaries in Switzerland and India. DyAnsys provides advanced medical diagnostic and monitoring systems to clinicians in individual practices and hospitals. DyAnsys, ANSiscope. First Relief, Primary Relief and Drug Relief are registered trademarks, of DyAnsys, Inc.
Website: www.dyansys.com
For more information, contact Srini Nageshwar, (888) 950-4321
SOURCE DyAnsys Inc.
These press releases may also interest you
|
News published on and distributed by: