Subjects: RCL, PSF
PADAGIS ISSUES VOLUNTARY NATIONWIDE RECALL FOR NITROGLYCERIN LINGUAL SPRAY DUE TO A POSSIBLE DEFECTIVE DELIVERY SYSTEM
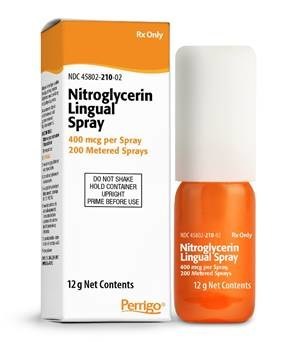
ALLEGAN, Mich., Dec. 27, 2021 /PRNewswire/ -- Padagis US LLC announced today it has issued a voluntary nationwide recall to the consumer/user level of the lots of Nitroglycerin Lingual Spray listed in the table below. Out of an abundance of caution, this product is being recalled from the market due to a complaint received that a unit may not dispense. There is a remote risk that the product may not properly dispense medication to patients in the event of a malfunction of their dispensing unit. This recall applies only to the 12g spray bottle and not the 4.9g spray bottle of this medication.
Drug | NDC | Strength | Net | Lot # | Expiration |
Nitroglycerin | 45802-210-02 | 400 mcg per | 12 g | 150892 | Oct 2022 |
153199 | Feb 2023 | ||||
156041 | Apr 2023 |
Risk Statement: If the product does not deliver the appropriate amount of nitroglycerin, the patient will likely continue to experience chest pain. The label advises that if relief is not obtained after 3 doses over 15 minutes the patient should promptly seek medical attention. To date, Padagis has not received any reports of adverse events related to this recall.
Nitroglycerin Lingual Spray is indicated for acute relief of an attack or prophylaxis of chest pain due to coronary artery disease in adult patients. All packaging and branding on affected units is that of Perrigo Company PLC. The product is packaged in a 12 g bottle contained within a carton. The medication was distributed Nationwide in the USA to wholesalers and retailers.
Padagis is notifying its distributors and customers by express package delivery service as well as electronic mail and is arranging for return of all recalled products. This recall is being directed at the consumer/user level. All customers, healthcare providers, and consumers are instructed to examine their inventory for Nitroglycerin Lingual Spray, 12g immediately and to quarantine, discontinue the distribution and use of, and return as directed all recalled lots of the product. Customers and healthcare providers are being provided recall information by Sedgwick Claims Management Services. All customers who have distributed this product to consumers have been requested to identify their customers and notify them immediately of this product recall. Healthcare providers, distributors, and retailers that have product which is being recalled should stop distribution. Patients who have this product should contact their healthcare provider for an alternate replacement before returning the recalled product. The necessary form to document product information, as well as other information, is available by contacting Sedgwick at [email protected] or 888-266-7912.
Patients with questions regarding this recall can contact 888-266-7912 M-F 8am ? 5pm EST. Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to using this product or any medical concerns.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
SOURCE Padagis
These press releases may also interest you
|
News published on and distributed by: