Subjects: CHI, FDA, DEI
Spark Aligners Does It Again With Market Leading Innovation And New FDA Approval To Give Doctors Greater Control And Flexibility
-- New Features in Release 11 Combined with Expanded Indication for Mixed Dentition to Treat Kids and Teens is a Home Run for Doctors --
BREA, Calif., June 16, 2021 /PRNewswire/ -- Ormco Corporation, a global leader of orthodontic solutions, today announced it has received FDA clearance to treat mixed dentition with its Sparktm Clear Aligner System, enabling orthodontists to treat younger patients. The clearance by the FDA makes Spark Clear Aligners one of a few doctor-directed aligners cleared in the U.S. for the treatment of younger patients. The robust Release 11 features new innovations and an improved user experience including new anatomical beveled attachments for patient comfort, redesigned bite ramps that enable doctors to customize for each specific tooth type to enhance engagement between the bite ramps and the teeth and downloadable STL files to offer doctors in-office solutions.
"With more than 75 percent of orthodontic case starts each year being kids and teens, we are excited that we can now enable doctors to offer the same innovative benefits of Spark treatment to younger patients," said Eric Conley, President of Spark Clear Aligners and Digital Orthodontics. "We are also confident the advancements of this new release will solidify Spark as the aligner of choice for orthodontists seeking great clinical outcomes and providing industry leading comfort and clarity for their patients."
Release 11 Key Features Enable Improved Clinical Outcomes, More Predictable and Efficient Treatment Planning & Greater Patient Comfort
-
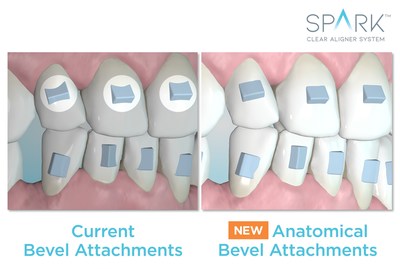
Anatomical beveled attachments conform to the surface of each tooth to provide a more uniform active attachment surface and to minimize sharp edges for patient comfort*.
"This enhances the product's appeal to the patient. You already have the best-performing plastic, clearest plastic, most stain-resistant plastic, and more comfortable edges on the market. Now, Ormco has enhanced the attachment comfort; you have the whole package," said Dr. Bill Dischinger**.
-
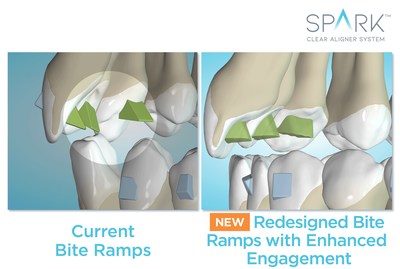
Redesigned bite ramps enhance the engagement between the bite ramps and the teeth and enable doctors to customize bite ramps for each specific tooth (incisors, canines, premolars) so that the active surface is oriented parallel to the occlusal plane. The new bite ramps have been lab tested for strength and durability*.
"Deep bite cases are known for their difficulty. Paralleled bite ramps will offer more predictable intrusive movement as they direct the force down the long axis of the tooth and will create less undesired tipping and friction points within the aligner leading to more efficient and predictable intrusion," said Dr. Trevor Nichols**.
-
Mixed detention support in the Approver software allows doctors to treat younger patients (kids and teens) with mixed dentition, including providing eruption guides for semi-erupted teeth and numbering primary teeth.
"This is a significant step to creating a Phase I/Phase II aligner approach to cases. Not only will Approver software enable doctors to better treat interceptive cases, but eruption guides will help control the eruption of the permanent dentition," said Dr. Trevor Nichols**.
-
Downloadable STL files of the first three stages and the last stage are now available for doctors to download from the Spark portal.
"With the ability to print the starter aligners, doctors can get a jump start on their cases and increase their case load and profitability. The last stage aligner allows the doctor and patient to have final models to use for retention for years to come," said Dr. Trevor Nichols. Dr. Nicole Scheffler** added, "This really is a game-changer for patients, doctors and treatment coordinators."
Spark Approver Software Enhancements
Spark Release 11 includes the following Approver software enhancements:
- Continued Evolution of CBCT integration based on doctor input. Spark Approver now features several new improvements for more seamless and effective integration of CBCT data for Spark Aligners. Additional diagnostic tools include a 2D clipping plane featuring multiple views, new tooth identification options, and enhanced CBCT performance and visual rendering.
- An enhanced tooth movement table now includes both crown and root movement values, as well as the addition of both tip and torque movement for intermediate and challenging movement.
- User interface improvements based on doctor input include a new preset for overjet, a new panel docking/undocking feature, redesigned and more friendly menu options, and simpler dialog boxes.
Enhancements for Easier Case Management
- New packaging improvements require less storage by including more aligners per box and reduces packaging waste. The introduction of connected aligner bags and Spark Aligners' packaging inserts will help ensure that aligner bags remain in order.
- More efficient workflow through staff accounts allows orthodontic team members the ability to submit and manage Spark Aligner patients through the Spark Portal and Approver software. Support for multiple billing addresses for practices that have multiple offices will be available in August 2021.
For more information on the Spark Aligner product and software enhancements, please visit ormco.com/Spark.
About the Spark tm Clear Aligner System
Sparktm Aligners are manufactured by Ormcotm, a global leader in innovative orthodontics products, with 60 years of orthodontics expertise, R&D and high manufacturing standards. Ormco has helped doctors create more than 20 million smiles in more than 130 countries. Spark Aligners offer the latest advancements in clear aligners and a better patient experience. Spark Approver 3D application and advanced clear aligner technology with TruGENtm material is designed to meet the needs of orthodontists, providing more efficient and effective tooth movement, compared to the leading aligner brand. Spark Aligners are designed to give orthodontists more control and flexibility so that they can more easily achieve healthy, confident smiles.
Trusted by orthodontists worldwide, Spark Aligners are clearer, more comfortable and stain less than the leading aligner brand. Spark Aligners are liked by patients: 100 percent of Spark patients surveyed would recommend Spark Clear Aligners to a friend.** For more information about Spark Aligners, visit www.sparkaligners.com.
# # #
|
*Data on file at Ormcotm |
|
** Dr. Bill Dischinger, Dr. Trevor Nichols and Dr. Nicole Scheffler are paid consultants of Ormco. The opinions expressed are those of Drs. Dischinger, Nichols and Scheffler. Ormco is a medical device manufacturer and does not dispense medical advice. Clinicians should use their own professional judgment in treating their patients. |
MEDIA CONTACT:
Sharon Scott, SparkPR@Secondlyco.com
310.594.6545


Video - https://mma.prnewswire.com/media/1532043/Ormco___Spark_Aligners.mp4
Photo - https://mma.prnewswire.com/media/1532039/Ormco___Spark_Aligners_Bevel_Attachment.jpg
Photo - https://mma.prnewswire.com/media/1532041/Ormco___Spark_Aligners_Bite_Ramps.jpg
Photo - https://mma.prnewswire.com/media/1532042/Ormco___Spark_Aligners_Mixed_Dentition.jpg
Logo - https://mma.prnewswire.com/media/1357605/Spark_Logo.jpg
These press releases may also interest you
|
News published on and distributed by:



