Subjects: BFA, FDA
SCONE Medical Receives FDA Emergency Use Authorization for Novel Aerosol Infection Containment Device
PHOENIX, Jan. 4, 2021 /PRNewswire/ -- As the world faces a new era of emerging and re-emerging infectious diseases, new technologies are paving the way for safer, more effective treatment options. The Self-Contained Negative Pressure Environment (SCONEtm) is a new technology developed by SCONE Medical Solutions Inc. (SMS) in collaboration with Mayo Clinic for infectious disease containment in hospitals. The FDA recently granted Emergency Use Authorization to the SCONEtm device on December 18, 2020.
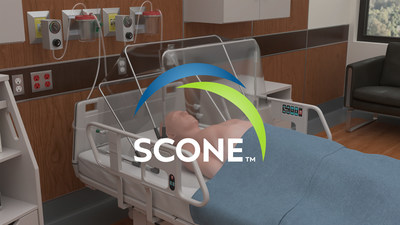
The highest risk of infectious transmission is from actively infected patients to health care workers (HCWs) during Aerosol Generating Procedures (AGPs), particularly in the acute care/triage setting. The SCONEtm is a small capacity, disposable device that uses negative pressure to vacuum out aerosols emitted around a patient's head and neck, adding an extra layer of "active" barrier protection for healthcare workers while treating potentially infectious patients. The SCONEtm can be quickly deployed for use and quickly disposed of after treatment.
Mike Adams, CEO of SCONE Medical Solutions Inc. (SMS) says, "We are pleased to collaborate with Mayo Clinic experts as we bring the SCONEtm device to market. The demand for barrier protection in hospitals has shown itself in a substantial way during this pandemic. The SCONEtm works not only as a protective barrier, but through the use of negative-pressure, actively reduces the spread of pathogenic aerosolized particulates that cause diseases like COVID-19."
During the spring of 2020, the SMS team ramped up efforts to begin designing, developing, testing the new SCONEtm device, and then in the fall prepared to launch a full-scale manufacturing process based in North Carolina. Now with the FDA EUA greenlight, SMS will begin distributing SCONEtm units across the country at the end of the year.
Dr. Brandon Lawrence, SCONE Chief Medical Officer and ER Physician in Phoenix, AZ, has been treating COVID-19 patients from the beginning. He states, "The widespread demand from HCWs for barrier protection devices, even the old ones without negative pressure, was overwhelming during the start of the pandemic. Adding negative-pressure technology allows patients to be more safely placed on CPAP/BIPAP, receive aerosolized nebulizer treatments, undergo emergent procedures if their COVID-19 status is unknown, and possibly allow end of life care visits with family."
According to Michael Wallace, M.D. at Mayo Clinic, who collaborated with the SMS team: "There is an urgent need for small capacity self-contained negative pressure environments that utilize existing hospital suction lines and HEPA filtration. The development of the SCONEtm device will provide new opportunities for hospitals to control aerosol spread to other patients and healthcare workers."
During the midst of this recent pandemic surge and beyond, SCONEtm aims to help ease the burden on hospitals by providing backup small-capacity negative pressure units that allow for safer treatment, triage, and patient transport. Implementing new, more sustainable, protocols using the SCONEtm device may also allow for planned family visitation and end-of-life care closure for those in desperate need of it most right now.
If you would like more information or to receive release updates, please visit www.sconemed.com, email [email protected] or call (855) 949-4997.
Mayo Clinic and Dr. Wallace have a financial interest in the technology referenced in this news release. Mayo Clinic will use any revenue it receives to support its not-for-profit mission in patient care, education, and research.
About SCONEtm Medical Solutions Inc.
SCONEtm reduces the spread of transmissible diseases through the use of Self-Contained Negative-Pressure Environments. The company uses technology developed with clinical support from Mayo Clinic to help hospitals protect their health care workers. Their low cost, disposable device is being manufactured in the United States for distribution beginning in October 2020.
SOURCE SCONE Medical Solutions Inc
These press releases may also interest you
|
News published on and distributed by: