Subjects: PDT, SCZ
CapsoVision Inc. Announces FDA Enforcement Discretion for At-Home Capsule Endoscopy Administration of CapsoCam Plus during COVID-19
SARATOGA, Calif., Sept. 23, 2020 /PRNewswire/ -- CapsoVision, an innovator in the gastroenterology diagnostics market, today announced that the U.S Food & Drug Administration (FDA) will apply enforcement discretion which allows at-home administration of the CapsoCam Plus® small bowel capsule endoscope during the COVID-19 pandemic for patients who are determined eligible for at-home administration.
The labeling addendum permits a fully remote capsule endoscopy procedure for eligible patients, eliminating the need for in-person interaction between clinic staff and patient.
"CapsoVision's advanced capsule technology delivers high-quality diagnostic images without creating a risk of in-person exposure to COVID-19," stated Johnny Wang, President and CTO. "Our team is proud to contribute during the pandemic and to continue to innovate within the emerging telehealth paradigm."
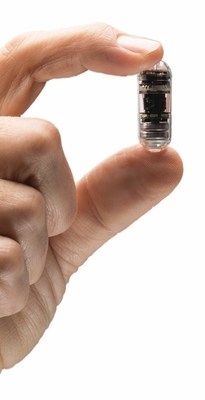
Along with its impressive 360° panoramic view, CapsoCam Plus is the only self-contained capsule endoscopy system that does not require external equipment that must be worn by the patient, returned to the clinic in 8-12 hours and disinfected between use. Patients simply ingest the capsule and return to their normal activities while the exam data is being captured. The images can then be easily reviewed by the physician via the CapsoCloud® cloud-based software, from anywhere there is internet access.
"The COVID-19 pandemic has challenged all physicians to rethink the way we deliver care now, and going forward," said Javier Parra, MD, Gastroenterologist in Miami, FL with Gastro Health." We need to do everything possible to continue to deliver healthcare in a timely and safe manner, while making an effort to reduce in-person contact and potential Coronavirus exposure. The CapsoCam Plus system will now allow us to offer this procedure to the appropriate patients efficiently and effectively, without the patient needing to visit the clinic. This is an added value to our practice and enables us to reduce exposure risks to our patients and staff while continuing the normal volume of office visits."
The CapsoCam Plus video capsule system is intended for the visualization of the small bowel mucosa in adults. It may be used as a tool in the detection of abnormalities of the small bowel
About CapsoVision, Inc.
CapsoVision, Inc. is a global medical device innovator located in Silicon Valley. A privately-held company with out-of-the-box thinking and top-notch talent, CapsoVision's mission is to empower physicians and patients with innovative technologies that improve diagnostic confidence and clinical outcomes. CapsoVision currently offers the CapsoCam Plus capsule endoscopy system in over 70 countries, and CapsoCloud® and CapsoProtm services in the USA. Visit www.CapsoVision.com for more information.
CapsoCam Plus® is also distributed by PENTAX Medical Company in the USA and Canada.
About Capsule Endoscopy
Small bowel capsule endoscopy is a non-invasive procedure in which a capsule with tiny camera(s) is swallowed by a patient and is used to visualize the middle part of the gastrointestinal tract (duodenum, jejunum and ileum).
SOURCE CapsoVision, Inc.
These press releases may also interest you
|
News published on and distributed by: