Subject: FDA
icotec ag Receives Approvals for Two KONG® VBR Spinal Systems Made with BlackArmor® and Ti-iT® in Europe and the US
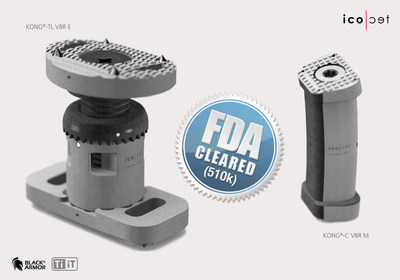
ALTSTAETTEN, Switzerland, June 18, 2020 /PRNewswire/ -- icotec ag announces that the KONG®-TL and the KONG®-C vertebral body replacement systems with the unique Titaniumcoating (Ti-iT®) receive FDA 510(k) clearance in the United States and CE approval in Europe .
"This is exciting news for icotec ag and our team as it allows us to expand our portfolio in multiple countries simultaneously, to include implants made from radiolucent, nonmetallic BlackArmor® material with a 360° osseoconductive Ti-iT® pure titanium coating" said Roger Stadler, CEO of icotec ag.
The surgical replacement of vertebral bodies is a common procedure after the removal of tumors from the spinal column or after a serious spinal fracture. A vertebral body replacement implant (VBR) is used to reconstruct and stabilize the spinal column. The surgical requirements for these stabilization procedures are met by the broad, innovative and technology platform developed by icotec ag.
- The fully modular design of these VBR systems with endplates in various profiles, sizes, angles and alignments complements the expandability afforded by the KONG®-TL VBR E (thoracolumbar) for the thoracic and lumbar spine providing surgeons with a great deal of flexibility in the surgical planning process. With the KONG®-C VBR for the cervical spine, the curved implant body ensures optimal adaptation to the anatomy of the patient.
- The 360° Ti-iT® pure titanium coating from icotec ag allows for the rapid engraftment of bone onto the implant thanks to the optimized osseoconductive structure.
- The unique icotec BlackArmor® Carbon/PEEK implant material enables artefact-free imaging to be carried out and, most notably, the improved planning, application, and follow-up care of radiotherapy for patients with tumors.
With over 15 years of clinical success and more than 40,000 implants made from the unique BlackArmor® material, icotec ag is the leading provider of Carbon/PEEK implants. As the global market leader in the surgical treatment of vertebral tumors, icotec ag has set a target of establishing a complete product portfolio that provides patients with improved therapy options. "With the imminent market launch of the KONG®-TL/C vertebral body replacement systems in Europe and the US, we have made significant strides toward achieving this target," commented Roger. He continues, "These systems perfectly complement the icotec ag pedicle screw systems already in surgical use."
Founded in 1999, icotec ag is a family-owned SME based in Altstaetten, Switzerland. icotec develops, manufactures, and distributes nonmetallic spinal implants made from BlackArmor®. icotec's proprietary BlackArmor® material is made up of continuous carbon fibers combined with polyether ether ketone (PEEK) and is manufactured with icotec's unique composite flow molding (CFM) injection molding manufacturing technology.
For further information, visit our website (www.icotec-medical.com).
SOURCE icotec Medical, Inc.; icotec ag
These press releases may also interest you
|
News published on and distributed by: