Subject: ERN
3SBio Unveils 2019 Annual Results: Revenue Rises by 16.0%, Normalized Net Profit attributable to owners of the parent Jumps by19.4%, R&D Expenses Soar 45.2%
HONGKONG, March 31, 2020 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today released its 2019 annual results, showing that the Company maintained steady growth, with core products continuously leading the market and more products being included into drug reimbursement lists. The Company has also been on track to advancing R&D pipelines, and stepping up efforts to introduce innovative therapies for cancer and autoimmune diseases into global markets. In the future, 3SBio will further boost its advantages with a comprehensive platform that integrates R&D, manufacturing, commercialization and investment cooperation, while consolidating and improving its status as a leading biopharmaceutical company.
Realizing sound business performance; sales of TPIAO exceeding RMB 2 billion
In 2019, 3SBio's revenue rose by 16.0% year on year to approximately RMB 5.318 billion. Gross profit increased by 18.5% to approximately RMB 4.393 billion. Normalized net profit attributable to owners of the parent added by 19.4% to approximately RMB13.92 billion.
The Company's four core products, including TPIAO, Yisaipu, EPIAO and SEPO, remained market leaders in China. Sales of TPIAO, which is the world's only commercialized recombinant human thrombopoietin ("rhTPO"), for the treatment of thrombocytopenia, soared 39.1% to exceed RMB 2 billion, with its market share jumping to 73.2%. Yisaipu, a product to treat rheumatoid arthritis , ankylosing spondylitis and psoriasis, had a market share of 60.9%. Two recombinant human erythropoietin ("rhEPO") products, EPIAO and SEPO, maintained market-leading positions, with their market share improving to 41.6%. As there is huge unmet demand for biologics in China, TPIAO and Yisaipu, which have low penetration rates, will see significant growth potential in the future.
Several of the Company's products and indications have been added into the updated 2019 National Reimbursement Drug List (NRDL), including Shinuo, a fluticasone propionate cream for the treatment of multiple skin diseases, the new indication of Yisaipu for the treatment of adult patients with severe plaque psoriasis, and the new indication for EPIAO for the treatment of anemia caused by chemotherapy for non-myeloid malignant tumors. Humulin, a mixed protamine zinc recombinant human insulin injection, was upgraded to Class A from Class B in the NRDL. Byetta, a therapy for the treatment of patients with type 2 diabetes, was added into the NRDL through negotiations.
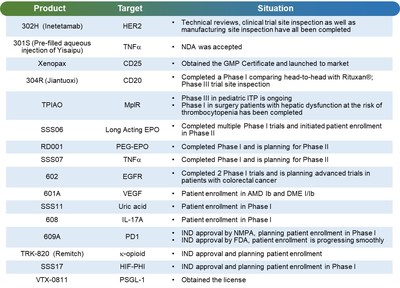
Also, Xenopax, the first approved recombinant humanized anti-CD25 monoclonal antibody injection in China, was granted the Chinese GMP certificate and launched in the market in October, 2019.
R&D expenses up 45.2%, with follow-up pipelines on 'fast track'
In 2019, 3SBio's R&D expenses soared by 45.2% to approximately RMB 527 million. The Company has its best-ever pipeline of biological cancer therapies, including anti-HER2, CD20, PD1, EGFR and VEGF antibodies. The Company's pipeline of biological therapies for the treatment of autoimmune and inflammatory diseases, including anti-TNFa, IL-17A and IL-5 antibodies, has all made significant progress.
302H (Inetetamab), an anti-HER2 monoclonal antibody drug, has completed the technical review, clinical trial site inspection as well as manufacturing site inspection. An application for manufacturing approval of pre-filled aqueous injection solution of Yisaipu has been filed and accepted by the National Medical Products Administration, and it is currently under the review process.
In 2019, the Company's drug candidates received five IND approvals, including: anti-PD1 antibody for the treatment of various cancers (simultaneous applications in China and the US); anti-IL-17A antibody for the treatment of moderate to severe plaque psoriasis; TRK820 (Remitch) for the treatment of pruritus in hemodialysis patients; and HIF-117 capsules for the treatment of anemia. In February 2020, the Company's anti-IL-5 antibody for the treatment of asthma was approved for a clinical trial.
The Company has also been proactively expanding new indications and second-generation products of existing products, including NuPIAO, a second-generation rhEPO; RD001, a pegylated long-acting rhEPO; and the pediatric ITP indication of TPIAO.
Also, the Company selected Verseau Therapeutics Inc's VTX-0811, a monoclonal antibody targeting PSGL-1 for the treatment of multiple types of cancer, as the first licensed program under the partnership in the field of immuno-oncology.
3SBio's R&D highlights in 2019
As of December 31, 2019, amongst the 32 product candidates within the Company's active pipeline, 22 were being developed as National New Drugs in China (including registration Class I and Biologics Class II), including 11 in oncology, 12 in autoimmune and other diseases, 6 in nephrology, 2 in metabolic diseases and 1 in dermatology.
Expanding global presence in therapies for cancer and autoimmune diseases
In 2019, 3SBio was continuously expanding external partnerships and global presence for its innovative therapies in the fields of cancer and autoimmune diseases, including the partnership with global biologics giant Samsung Bioepis in South Korea for the development of biosimilar candidates; collaboration with Verseau Therapeutics in the United States for global clinical development of macrophage checkpoint modulators; partnership with Taiwan Liposome Company for the development of innovative liposomal products; and collaboration with Numab to develop new multispecific antibodies for cancer immunotherapy. The Company also invested GenSight and Sensorion to explore innovative gene therapies for ophthalmic diseases and innovative treatments for inner ear diseases.
In early 2020, the Company became a limited partner in the MPM Oncology Innovations Fund (INV), and agreed to make donation to support early-stage oncology research at Dana-Farber Cancer Institute, a world-leading cancer research and treatment center.
These collaborations demonstrated 3SBio's excellent expertise in international development and operation, while laying a key stepping stone for its future globalization strategy. The Company will continue to pursue selective mergers and acquisitions and collaboration opportunities and explore cutting-edge innovative therapies in early stages, with an aim to enrich its existing product portfolio and become a leader in next-generation immuno-oncology therapies.
Comprehensive platform with strong competitive advantages
In the future, 3SBio intends to reinforce its position as a leading biopharmaceutical company in China by continuously leveraging its integrated R&D, manufacturing, commercialization and investment cooperation platforms. The Company will also focus on developing innovative biologics products to address unmet medical needs to benefit more patients.
The Company will fully integrate the R&D teams of nearly 400 people on multiple R&D platforms, and actively develop innovative therapies including monoclonal antibodies, bispecific antibodies, antibody fusion proteins, and cell therapies, thereby bringing a variety of treatment options to patients. The Company will kick off multiple phase III clinical trials this year, file new drug applications for more than 10 products in the next 3 years, and also submit IND applications for10-15 new monoclonal antibodies and bispecific antibodies (simultaneous applications in China and the US).
The Company has approximately 38,000-liter capacity in mAb facility, mammalian cell-based, bacteria cell-based and small molecule manufacturing facilities, and more than 27 years of experience in manufacturing biologics medicines. With large-scale production capacity that meets international quality standards, the Company is able to continuously supply the market with high-quality biologics. Over nearly 3 decades, the Company has been well recognized for its strong commercial operation capabilities and sales network throughout the country, which supports its sustainable growth.
Dr. Jing LOU, Chairman and CEO of 3SBio, commented: "Under the backdrop of the COVID-19 pandemic globally, 3SBio supports the country in our own way, through thick and thin. As we face a market environment where opportunities and challenges coexist, 3SBio is still maintaining strong growth momentum by leveraging our well-established systems that we've developed over the years. we will strive to overcome all difficulties, accelerate clinical applications and progress for our pipeline. We will also expand our production capacity and give full play to the advantages of our integrated platform. We aim to become a globally leading Chinese biopharmaceutical company, and continuously improve the availability of innovative biologics to benefit more patients."
SOURCE 3SBio Inc.
These press releases may also interest you
|
News published on and distributed by: