Subjects: PDT, FDA
FDA Clears Fluidda's Broncholab Platform for Use in Clinical Practice
LOS ANGELES, March 11, 2020 /CNW/ -- The US Food and Drug Administration provided market clearance to Fluidda for its Broncholab platform. Broncholab provides a number of Functional Respiratory Imaging (FRI) parameters to physicians via an online platform to assist in diagnosing and monitoring of respiratory diseases.
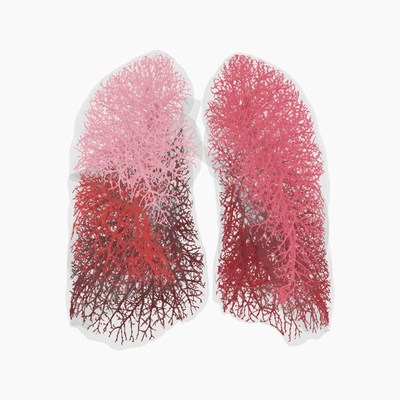
The severity of respiratory diseases is often underestimated by conventional lung function tests, such as spirometry. As healthy areas in the lung tend to compensate for sick areas, spirometry can indicate normal lung function despite declining lung health. Quantitative HRCT techniques such as FRI yields more accurate regional information that is clinically relevant for early and correct diagnosis and to optimize treatments for individual patients.
Dr. Jan De Backer, Fluidda's CEO, states: "Functional Respiratory Imaging has been used in clinical trials for many years and has proven its value time and time again. Broncholab now extends these capabilities into clinical practice which is a tremendous step forward in our quest for better respiratory care. We are living in a time where respiratory viruses cause significant disruption to daily life with high associated cost. We are striving to better understand respiratory illnesses with our novel technology to be more prepared for the next viral outbreak and to deal with the increased number of patients with lung diseases worldwide."
About Fluidda
FLUIDDA is the world leader in the field of Functional Respiratory Imaging (FRI). This technique combines HRCT scans, Computational Fluid Dynamics and Artificial Intelligence technology, which offers vast improvements by making clinical trials shorter, faster and thus, more cost effective. FRI also helps patients and healthcare providers in offering a unique entry point in personalized medicine, by optimizing diagnosis, monitoring disease progression and the effects of therapy. Fluidda is both a clinical research services company and a partner of healthcare professionals by offering worldwide evidence for better respiratory treatment.
Logo - https://mma.prnewswire.com/media/1122610/Fluidda_Logo.jpg
Photo - https://mma.prnewswire.com/media/1122611/Fluidda_FRI_Tech.jpg
SOURCE Fluidda
These press releases may also interest you
|
News published on and distributed by: