Subjects: PDT, TRI
Algentech Granted US Patent For A Key Genome Editing Technology
VERSAILLES, France, Jan. 20, 2020 /PRNewswire/ -- ALGENTECH, announces the issuance of a key patent in the field of genome editing by the U.S. Patent Office (U.S. patent number 10457950). The technology claimed by the patent amplifies the effectiveness of gene editing in eukaryotic cells and can be applied in synergy with nucleases, including zinc finger nucleases, TALENs, Crispr-Cas9...
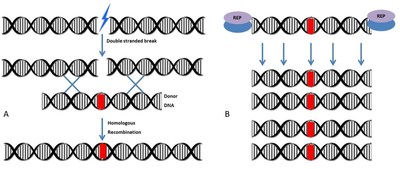
Algentech's invention optimizes the cellular process known as homologous recombination. In order to precisely insert or modify a genetic sequence, existing technologies use nucleases that introduce specific breaks in the target DNA and then rely on the cellular machinery to complete the editing process. The mechanism is simple: the DNA fragment (donor DNA) carrying the genetic modification or sequence to be inserted has at its ends sequences homologous to the DNA strand to be modified. This similarity around the cutting site is recognized by the cellular machinery: the strands assemble. The natural DNA repair process then repairs the cut zone, using the donor DNA fragment as a matrix, inserting it into the target DNA. However, the effectiveness of homologous recombination repair remains low.
Figure 1. (A) The double ?stranded breaks induced on the genome by nucleases are repaired by a homologous recombination mechanism that occurs between genomic DNA and donor DNA (B) A large number of donor DNA molecules are produced by Algentech's patented replication system (REP), thus contributing to the effectiveness of homologous recombination.
ALGENTECH's invention significantly increases the efficiency of the homologous recombination process by producing two modules: (1) a multi-domain protein capable of binding to the fragment to be inserted and to the target DNA; (2) a donor DNA amplification vector that relies on a viral replication system. The technique produces a large amount of donor DNA strands, thus multiplying the efficiency of modifications introduced: a technological leap that finds applications in crop improvement, gene therapy and synthetic biology.
"The issuance of this patent in the United States in the field of nuclear genome editing is a major step in our strategy and strengthens our intellectual property portfolio in a sector that has revolutionized genomics in recent years," says Alexander Sorokin, President of Algentech.
About ALGENTECH
ALGENTECH has developed proprietary molecular tools for editing the genome of eukaryotic cells. In addition to new methods of nuclear genome editing, ALGENTECH is a pioneer in the transformation of the mitochondrial genome and the development of self-replicative molecules for multigenic expression in chloroplasts with vast fields of application, particularly in synthetic biology.
The company offers its customers unique technologies tailored to their specific applications in the form of service delivery and licensing agreements. ALGENTECH is a member of the Genopole Cluster of Biotechnology companies (Evry, France) and is supported by Scientipôle IDF Capital, BADGE, Hedera and Investessor.
Contact Details:
Isabelle Malcuit
[email protected]

SOURCE ALGENTECH
These press releases may also interest you
|
News published on and distributed by:



