Subject: FDA
Shimadzu Medical Systems receives FDA 510(k) for the FLUOROspeed X1 RF system
TORRANCE, Calif., Aug. 12, 2019 /PRNewswire/ -- Shimadzu Medical Systems USA, a subsidiary of Shimadzu Corporation, is proud to announce that they have received FDA 510(k) clearance for the FLUOROspeed X1, patient side conventional RF table system.
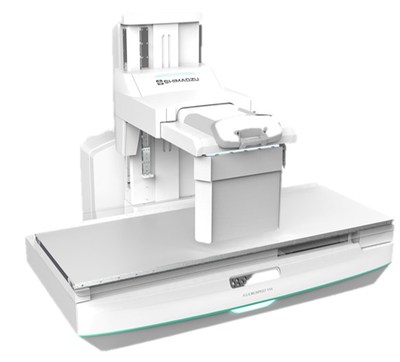
Shimadzu Medical Systems USA (SMS) has introduced a new radiographic/fluoroscopic (RF) system called the FLUOROspeed X1, a conventional RF table system offering high image quality and a multitude of features that improve work flow and operator efficiencies which contributes to lower cost of care.
As the newest U.S. based product in the FLUOROspeed series, the FLUOROspeed X1 with its 665 lb. static patient weight and 500 lb. all motion weight, easily performs both bariatric and routine daily fluoroscopic and radiographic exams. "The X1 is an outstanding RF system offering a cost-effective balance of functionality to support a wide range of general RF applications, such as chest, abdomen, or extremities along with Upper GI's, modified swallows and even joint injections," says Charles Cassudakis, Director of Radiographic and RF modalities for SMS. "An ambidextrous control handle for the imaging deck along with fingertip access to APR's, image recording functions and site-specific programmable function buttons, are all standard on the new X1 RF system, all improving room workflow," continues Cassudakis.
Jim Mekker, Product Director for SMS emphasizes that, "The X1 was designed and built from years of collecting user feedback specifically from and for the US marketplace and the result is a remarkable, user friendly and highly effective RF system with exclusive features like a park anywhere imaging deck. Additionally, the X1 is built with Shimadzu's world renown durability and reliability. This system belongs in every X-Ray department nationwide."
The FLUOROspeed X1 conventional RF system, designed with patient side table controls for the operator, is practically priced and comes equipped with a 17"x17" dynamic digital X-ray detector (FPD) in the table bucky allowing it to both be used for fluoroscopy as well as radiographic exams. With its 31.5-inch aperture opening between table top and deck, the X1 is the ideal digital RF system providing access for imaging patients in wheelchairs, yet it can fit in smaller rooms where space is limited. Furthermore, by adding a second X-ray tube on an overhead rail, the system functionality and versatility of the room increases exponentially.
The FLUOROspeed X1 received FDA 510(k) clearance on August 5th, 2019 and is now available for sale throughout the US.
About Shimadzu
Shimadzu Corporation, founded in 1875 in Kyoto, Japan and the parent of Shimadzu Medical Systems USA (SMS), is a global provider of medical diagnostic equipment including conventional, interventional and digital X-Ray systems. Shimadzu Medical Systems USA is headquartered in Torrance California with Sales and Service offices throughout the United States, the Caribbean and Canada. Its sales and marketing office is located in Cleveland, Ohio, and its direct operations has headquarters in Dallas, Texas. Visit Shimadzu Medical Systems USA at www.shimadzu-usa.com or call (800) 228-1429.
For further information contact:
Frank Serrao, Marketing Manager
(800) 228-1429
[email protected]

SOURCE Shimadzu Medical Systems USA
These press releases may also interest you
|
News published on and distributed by:



