Subject: FDA
Soom Launches Mobile App That Notifies Patients, Caregivers and Nurses of Medical Device Recalls
BOSTON, July 15, 2019 /PRNewswire/ -- Soom, a pioneer in utilizing barcode and knowledge graph technologies to bridge information gaps between data sources and physical products, has introduced SoomSafety, an iOS mobile app that allows users to scan a medical device and receive instructions for use, safety and recall information directly from the device manufacturer and U.S. Food and Drug Administration (FDA).
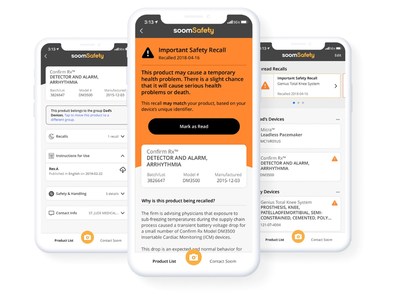
"We built SoomSafety to help patients and caregivers relying on implanted medical devices and using medical devices at home answer one critical question, 'Is this medical device safe to use?'" said Charlie Kim, President and CEO of Soom. "Our technology makes it possible to connect previously siloed medical device data, giving patients?and their caregivers?more proactive control over their health and safety."
SoomSafety is the first app to utilize openFDA, open-source databases that enable developers to quickly and easily use FDA data in applications. "This year alone, 26 medical device products have been recalled, affecting nearly 50 million individual devices in the United States." According to Kim, many patients are never informed of these recalls due to incomplete information in the medical device supply chain. Soom is empowering patients and caregivers to proactively manage their medical devices for improved patient outcomes and peace of mind.
SoomSafety users scan the barcode on a medical device, such as an insulin pump, nebulizer or apnea monitor, to automatically identify the device and store it in the app. The app also identifies and stores implanted medical devices like artificial joints, pacemakers and heart valves by scanning the barcode on a patient's medical device identification card.
Once a device is stored, the app checks for FDA recall information, provides next steps in the event of a recall and pushes notifications if the device is ever recalled. In addition, the app displays safety and use information for each stored device.
The idea for SoomSafety was prompted by Kim's personal experience with a medical device recall that threatened the life of his youngest daughter.
"I've experienced first-hand what it feels like to wonder if a medical device that your loved one uses?relies on?is safe," said Kim. "It's a feeling that no patient, parent or caregiver should have to endure. That's why at Soom we're dedicated to finding new ways to use technology to ensure clarity and confidence in the medical devices we use."
SoomSafety is available now for free in the Apple App Store. Patients, caregivers and homecare professionals can learn more about SoomSafety at www.soom.com/soomsafety.
About Soom
Soom uses proven technology and science to enable interoperability, safety, collaboration and efficiency throughout the healthcare value chain. Headquartered in Boston, with offices worldwide, and a pioneer in the industry, we offer a cloud-based enterprise SaaS platform that provides the healthcare industry with a simple solution that enables master data accuracy, data governance, error correction, and, ultimately, improved patient safety. The Soom platform is a powerful knowledge graph connecting siloed databases and is compliant with GS1 standards and global UDI regulations. For more information, visit www.Soom.com or follow us on Twitter, LinkedIn and Facebook.
SOURCE Soom
These press releases may also interest you
|
News published on and distributed by:



