Subjects: TRI, FDA
icotec AG Granted FDA Clearance To Market VADER®one Pedicle Screw System
ALTSTAETTEN, Switzerland, July 8, 2019 /PRNewswire/ -- icotec AG, a Swiss company, today announced the VADER®one pedicle screw system was granted U.S. Food and Drug Administration (FDA) 510(k) clearance for both minimally-invasive and open spine surgical procedures. VADER®one, made from icotec's unique BlackArmor® material, was designed for secure stabilization and post-operative visualization, which is important after spinal tumor procedures.

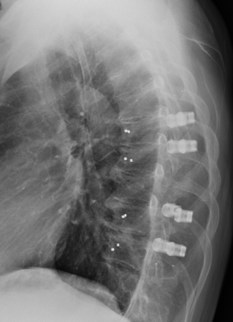
In a recent publication, the neurosurgery and radiation oncology group from Technical University of Munich, Germany, stated: "In terms of postoperative evaluation of residual or recurrent tumor within the periphery of the spinal canal and column and for accurate radiation planning, CFRP* implants hold major advantages when compared with standard titanium implants by generating significantly fewer image artifacts." [1]
VADER®one is made from icotec's unique BlackArmor® Carbon/PEEK material, which consists of continuous carbon fibers combined with PEEK (polyetheretherketone), and is produced using icotec's injection molding CFM (Composite Flow Molding) manufacturing technology. This process allows for 3-dimensionally reinforced, nonmetallic implants providing both, the strength and radiolucency needed for oncology patients and their treatment.
icotec is the only company manufacturing spinal implants made of radiolucent BlackArmor® Carbon/PEEK composite material. Implants made of BlackArmor® are biocompatible and have been successfully implanted for over 15 years. Their X-ray, CT and MRI translucency makes a significant difference during the surgical procedure, and postoperatively in assessing the site of care. In patients with spinal tumors, optimal delineation of the tumor from healthy tissue can facilitate radiotherapy planning, improve radiosurgery treatment and allow immediate and precise monitoring of possible relapses (tumor recurrence).
"Our ability to use icotec's BlackArmor® material for stabilizing the spine offers advanced-stage tumor patients the ability to consider a larger variety of treatment options with improved therapy planning, dose delivery and follow-up evaluation," says Roger Stadler, CEO of icotec AG, Switzerland. "It is a great achievement for our company and our employees to obtain FDA clearance, a significant milestone in our company's history."
The VADER®one implants are intended to restore the integrity of the spinal column even in the absence of fusion for a limited time period in patients with advanced stage tumors involving the thoracic and lumbar spine in whom life expectancy is of insufficient duration to permit achievement of fusion.
icotec AG is a family-owned SME in Altstaetten, Switzerland established in 1999. icotec designs and manufactures nonmetallic spinal implants made from BlackArmor® (Carbon/PEEK). More than 10'000 icotec BlackArmor® pedicle screws have been implanted in countries outside the USA since their market introduction.
For more information, visit our website www.icotec-medical.com or contact us at [email protected]
* CFRP (carbon fiber-reinforced polyetheretherketone) is identical to Carbon/PEEK.
[1] Ringel, F., et al., Radiolucent carbon-fiber reinforced pedicle screws for the treatment of spinal tumors: Advantages for radiation planning and follow-up imaging. World Neurosurg, 2017.
SOURCE Icotec Medical
These press releases may also interest you
|
News published on and distributed by:



