Subjects: PDT, TRI, FDA
Zebra Medical Vision Receives FDA Approval for World's First AI Chest X-ray Triage Product
KIBBUTZ SHEFAYIM, Israel, May 13, 2019 /PRNewswire/ -- Zebra Medical Vision (https://www.zebra-med.com/), the deep learning imaging analytics company, today announces that it has received FDA 510(k) clearance for HealthPNX - an AI alert for pneumothorax (PNX), based on chest X-rays.

The latest FDA clearance, received in May 2019, focuses on an AI alert for "stat" (urgent) findings of pneumothorax and demonstrates a promising potential to substantially reduce turnaround time and increase the radiologist's confidence in making this diagnosis. Chest X-rays are one of the world's most used imaging modalities.
Pneumothorax is a condition in which there is an accumulation of gas within the pleural space between the lung and the chest wall. Without prompt management, pneumothorax can lead to total lung collapse and other potentially fatal complications. This condition is most commonly diagnosed by a chest X-ray scan, though it is one of the hardest to interpret, and is known for high disagreement rates even between experienced radiologists. Misdiagnosis or late-diagnosis of Pneumothorax impacts around 74,000 Americans per year.
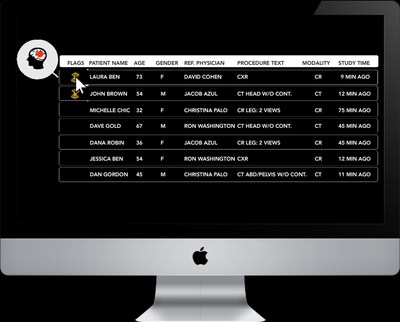
The patent pending technology automatically detects findings suggestive of Pneumothorax based on CXR or digital radiography (DR) scans, and alerts the medical team. In hospitals where Zebra-Med's "All in one" (AI1) solution is integrated into the radiologist's worklist, the scan is flagged so that the radiologist can address it in a timely manner. This first of its kind FDA cleared solution can save physicians more than 80% of the time taken to reach the acute condition, compared to the traditional First In First Out (FIFO) methodology.
The Pneumotorax product is a result of the extensive work accomplished by the Zebra-Med's research lab. The chest X-ray AI network was trained using millions of images to identify over 40 common clinical findings. The results of the Textray study establish a new bar for AI research in medical imaging, demonstrating high rates of agreement between the algorithm and human radiologist experts.
"We are happy to add this important capability to our All-in-One (AI1) package and add more value to busy radiology departments," says Eyal Gura, Zebra-Med's CEO and Co-Founder. "Health providers across the U.S. that already use the many Zebra-integrated PACS and worklist systems, will be able to easily deploy our triage solution and improve their patients' care and outcomes".
"In a clinical validation study we performed, Zebra-Med's acute CXR pneumothorax and CT Brain bleed products demonstrated a promising potential to substantially reduce turnaround time and increase the radiologist's confidence in making these diagnoses," said Dr. Terence Matalon, Chairman of Imaging at Albert Einstein Medical Center. Zebra-Med's AI1 Triage Solution is the first of its kind for both CTs and X-rays, and currently addresses two acute conditions: intracranial hemorrhages (head CTs, FDA pending), and pneumothorax (chest X-rays).
Zebra-Med's previous FDA approval, received in July of 2018, focuses on coronary calcium scoring algorithm, which can detect coronary artery disease (CAD). The growing number of regulatory approvals within Zebra-Med's AI portfolio allows the company to continue to enter new markets. With nine CE marked products and more FDA pending applications in the pipeline, Zebra-Med will continue to expand its footprint in the United States, and release additional automated solutions worldwide, to help radiologists and health providers produce more comprehensive, accurate outcomes, without compromising the quality of care.
Read more about the X-ray product team journey here.
About Zebra Medical Vision
Zebra Medical Vision's Imaging Analytics Platform allows healthcare institutions to identify patients at risk of disease and offer improved, preventative treatment pathways to improve patient care. Zebra-Med was founded in 2014 by Eyal Toledano, Eyal Gura, and Elad Benjamin and funded by Khosla Ventures, Marc Benioff, Intermountain Investment Fund, OurCrowd Qure, Aurum, aMoon, Nvidia, J&J, and Dolby Ventures. Zebra Medical Vision has raised $50 million in funding to date, and was named a Fast Company Top-5 AI and Machine Learning company.
Company Media Contact:
Alona Stein
Blonde 2.0 for Zebra Medical Vision
[email protected]
+972-507782344

SOURCE Zebra Medical Vision
These press releases may also interest you
|
News published on and distributed by:



