Subjects: PDT, FDA
CarboFix's Extends its CarboClear® Carbon Fiber Pedicle Screw System Product Line with its FDA Cleared Trans-Connector
DOVER, Delaware, Oct. 3, 2018 /PRNewswire/ -- CarboFix in Orthopedics LLC., has announced today that the U.S. Food and Drug Administration (FDA) has cleared its CarboClear® Carbon Fiber Transverse Connectors to be used in conjunction with its Carbon Fiber Pedicle Screw System, a novel device to surgically treat oncological patients, cleared by the FDA earlier this year.
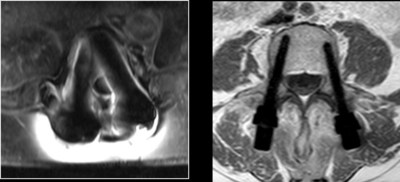
Carbon fiber implants are proposing unique advantages to the oncological patients and their physicians. Among those advantages are: enhanced radiation therapy planning abilities, allowing radiation treatment to optimize the radiation dose to the tumor with minimal collateral tissue damage, and enhanced follow up abilities due to artifacts-free CT/MRI. In addition, unparalleled fatigue strength to support the impaired healing process in those patients, as well as compatibility with particle radiation (proton and carbon ion) and other stereotactic radio-surgery modalities.
Dr. Carl Lauryssen, director of neurosurgery at St. David's Round Rock Medical Center, TX, one of the first users of the CarboClear® System in the US, said: "These unique implants provide enhanced imaging follow-up and radiation treatment. These implants are a game changer for spinal oncology cases." Ron Szekely, CarboFix's VP Sales and Marketing, said: "This is another major breakthrough for CarboFix in its endeavor to bring the next revolution, using carbon fibers as the material of choice in orthopedic implants. We will continue to broaden our portfolio for the spine as we did with our full range trauma line."
About CarboFix in Orthopedics LLC.
CarboFix in Orthopedics LLC, is the world leading company in developing, manufacturing and marketing innovative carbon fiber spine solutions. The company products are cleared by the FDA and other regulatory authorities, are CE marked, and are being sold worldwide.
For more information, please visit www.Carbo-fix.com.
For More Information Contact:
Ron Szekely
[email protected]
+97-254-234-6033
SOURCE CarboFix in Orthopedics LTD.
These press releases may also interest you
|
News published on and distributed by:



