Subject: FDA
PARI Pharma's eFlow® Technology device, LAMIRAtm, approved as the only nebulizer system to deliver Insmed's ARIKAYCE® (amikacin liposome inhalation suspension)
STARNBERG, Germany, Oct. 3, 2018 /PRNewswire/ -- PARI Pharma GmbH, a company focused on the development and commercialization of advanced aerosol delivery systems based on eFlow Technology, announces approval of its optimized eFlow technology nebulizer LAMIRAtm together with Insmed's ARIKAYCE® (amikacin liposome inhalation suspension). The drug/device combination product received U.S. Food and Drug Administration (FDA) accelerated approval on September 28th, 2018 under a New Drug Application.
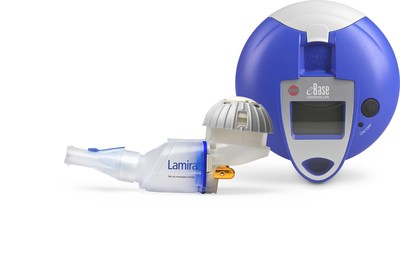
LAMIRA, designed, optimized, and developed by PARI Pharma, is intended only for use with ARIKAYCE and the treatment of MAC lung disease as part of a combination antibacterial drug regimen for adult patients with limited or no alternative treatment options. ARIKAYCE is the first FDA approved inhaled liposomal suspension which is aerosolized using a vibrating membrane nebulizer. "LAMIRA is another great example of an eFlow Technology nebulizer which we optimized for the administration of a specific drug formulation, indicating the strong capabilities of the eFlow Technology platform," states Dr. Martin Knoch, President of PARI Pharma. "It is rewarding to bring, in close collaboration with the team at Insmed, such technical advancements into the hands of patients with little to no alternative treatment options," adds Dr. Knoch.
LAMIRA to deliver ARIKAYCE is PARI Pharma's 3rd FDA approval where an eFlow Technology device was used exclusively to deliver the approved drug.
About MAC Lung Disease
Mycobacterium avium complex (MAC) lung disease is a rare and serious disorder that can significantly increase morbidity and mortality. Patients with MAC lung disease can experience a range of symptoms that often worsen over time, including chronic cough, dyspnea, fatigue, fever, weight loss, and chest pain. In some cases, MAC lung disease can cause severe, even permanent damage to the lungs, and can be fatal.
MAC lung disease is an emerging public health concern worldwide with significant unmet needs. Current guideline-based treatment involves the use of multi-drug regimens that are not specifically approved for MAC lung disease. The course of treatment is often two years or more and is inadequate in treating the disease in many patients.
About ARIKAYCE® (amikacin liposome inhalation suspension)
ARIKAYCE is the first and only FDA-approved therapy indicated for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen for adult patients with limited or no alternative treatment options. ARIKAYCE is a novel, inhaled, once-daily formulation of amikacin, an established antibiotic that was historically administered intravenously and associated with severe toxicity to hearing, balance, and kidney function. Insmed's proprietary Pulmovancetm liposomal technology enables the delivery of amikacin directly to the lungs, where it is taken up by lung macrophages where the infection resides. This approach prolongs the release of amikacin in the lungs while limiting systemic exposure. ARIKAYCE is administered once daily using the LAMIRA Nebulizer System manufactured by PARI Pharma GmbH.
About PARI Pharma and eFlow® Technology
PARI Pharma is a world leader in the development of aerosol delivery devices optimized with drug products for nebulized treatments using its proprietary technologies and expertise. PARI's platform of nebulizer systems based on eFlow Technology incorporate a vibrating, perforated membrane. eFlow Technology devices are designed to significantly improve upper and lower respiratory tract deposition and reduce the burden of treatment for patients with severe respiratory conditions. PARI Pharma, a PARI Medical Holding company, is located near Munich, Germany with a major presence in the United States. For additional information, please visit www.paripharma.com.
Arikayce® is a registered trademark of Insmed Incorporated.
Lamiratm is a trademark of PARI Pharma GmbH.
eFlow® is a registered trademark of PARI Pharma GmbH.
Contact: | Michael Hahn |
Director eFlow Partnering & Strategy | |
PARI Pharma GmbH | |
+49 (0) 89 74 28 46 -831 | |
SOURCE PARI Pharma
These press releases may also interest you
|
News published on and distributed by:



