Subject: FDA
FDA Clears Augmented Reality Visualization Solution GLOW800
BUFFALO GROVE, Ill., Sept. 20, 2018 /PRNewswire/ -- Leica Microsystems received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its augmented reality GLOW800 surgical fluorescence for vascular neurosurgery. In combination with ICG (Indocyanine Green), GLOW800 allows surgeons to observe cerebral anatomy in natural color, augmented by real-time vascular flow in a single image, with full depth perception. This augmented reality solution provides the surgeon a complete view of anatomy and physiology to support crucial decisions and actions during vascular neurosurgery.
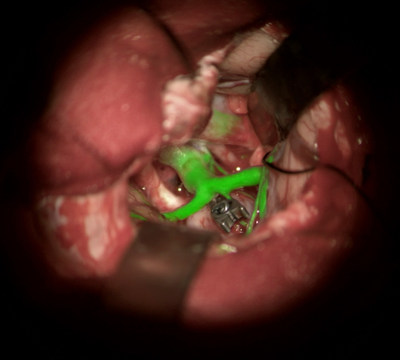
"For the past decade Leica Microsystems has been pioneering new fluorescence imaging technologies in partnership with surgeons to advance surgical practices," says Markus Lusser, President of Leica Microsystems. "GLOW800 and future modalities based on the GLOW AR platform will allow surgeons to perform life-changing neurosurgical interventions with the confidence that they have the best possible visual information right in the field of view."
GLOW800 AR fluorescence is the first of many imaging modalities that will be based on the GLOW AR platform from Leica Microsystems. GLOW AR modalities can be fully integrated in the ARveo digital augmented reality microscope which launched earlier this year. Following the FDA 510(k) clearance of GLOW800, ARveo customers in the U.S. can now experience the full advantages of augmented reality visualization in the operating room.
"Leica Microsystems is a company that works closely with neurosurgeons to bring new technologies to market that really respond to our needs," says Cleopatra Charalampaki, Professor of Neurosurgery, Cologne Medical Center, Germany. "GLOW800 AR is an exciting new approach which provides a totally new view during vascular neurosurgery. I have excellent spatial orientation and I am impressed with the crisp delineation of vessels. I believe GLOW800 AR fluorescence will have a significant impact on surgical outcomes in the future."
About Leica Microsystems
Leica Microsystems develops and manufactures microscopes and scientific instruments for the analysis of microstructures and nanostructures. Ever since the company started as a family business in the nineteenth century, its instruments have been widely recognized for their optical precision and innovative technology. It is one of the market leaders in compound and stereo microscopy, digital microscopy, confocal laser scanning microscopy with related imaging systems, electron microscopy sample preparation, and surgical microscopes.
Leica Microsystems has seven major plants and product development sites around the world. The company is represented in over 100 countries, has sales and service organizations in 20 countries, and an international network of distribution partners. Its headquarters are located in Wetzlar, Germany.

SOURCE Leica Microsystems
These press releases may also interest you
|
News published on and distributed by:



