Subjects: CHI, RCL
Tris Pharma, Inc. Expands Its Voluntary Nationwide Retail Recall of Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 mL, Due to Higher Concentration of Ibuprofen
MONMOUTH JUNCTION, N.J., Jan. 29, 2019 /PRNewswire/ -- Tris Pharma, Inc. is expanding the scope of its November 2018 recall by adding three (3) additional lots of Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 mL, to the retail (pharmacy) level. Some units from these batches have been found to have higher levels of Ibuprofen concentration.
Infants already susceptible to the adverse effects of ibuprofen may be at a slightly higher risk if they receive medication from an impacted bottle. There is a remote probability that infants, who may be more susceptible to a higher potency level of drug, may be more vulnerable to permanent NSAID-associated renal injury. Some units from these six (6) lots have been found to contain Ibuprofen as high as 10% above the specified limit. Studies have shown that safety issues or toxicity is generally accepted to be a concern in infants at doses in excess of 700% of the recommended dose.1 To date, no serious adverse events have been reported related to this recall.
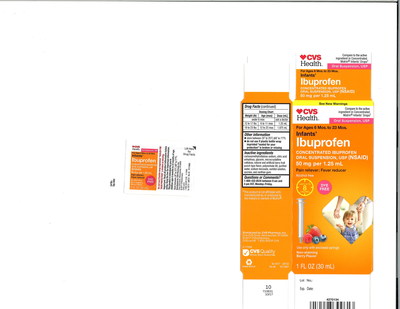
The product is used as a pain reliever/fever reducer and is packaged in ˝ oz. and 1 oz. bottles. This voluntarily recall includes the six (6) lots listed below:
Lot No. | NDC | EXPIRATION | DESCRIPTION | COMPANY |
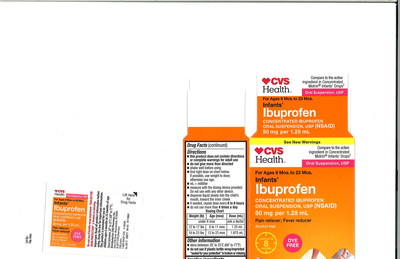
4718 | 59779-925-23 | 12/19 | CVS Health: Infants' | CVS |
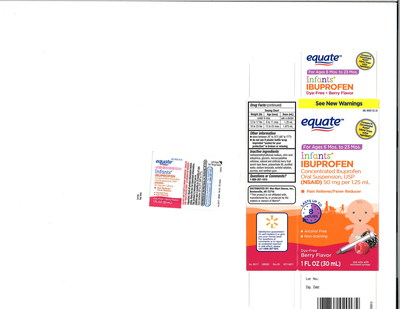
00717005A | 49035-125-24 | 02/19 | Equate: Infants' | Wal-Mart |
00717006A | 59779-925-24
(Labeled as: | 02/19 | CVS Health: Infants' | CVS |
00717009A (Previously | 49035-125-23 | 02/19
| Equate: Ibuprofen Oral | Wal-Mart |
00717015A (Previously
| 49035-125-23 | 04/19
| Equate: Ibuprofen Oral | Wal-Mart |
00717024A (Previously | 49035-125-23 | 08/19 | Equate: Ibuprofen Oral | Wal-Mart |
59779-925-23 | CVS Health: Ibuprofen USP, 50 mg per 1.25 | CVS | ||
55319-250-23 | Family Wellness: USP, 50 mg per 1.25 | Family |
Tris Pharma, Inc. manufactures Ibuprofen Oral Suspension Drops, USP for a single customer, who markets and distributes the product to retailers. The retailers should stop further distribution of the affected lots, which are being recalled. Tris Pharma, Inc. has notified its customer by urgent recall notice and has arranged for the return of recalled products from retailers and distributors.
Consumers with questions regarding this recall may contact Tris Customer Service by 732-940-0358 (Monday through Friday, 8:00 AM ET- 5:00 PM PT) or via email at [email protected]. Consumers, who may be concerned, should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration (FDA). The FDA has approved a class II retail level recall for this recall.
1. Hall AH, Smolinske SC, Conrad FL, et al. Ibuprofen overdose: 126 cases. Ann Emerg Med 1986; 15:1308.
SOURCE Tris Pharma, Inc.
These press releases may also interest you
|
News published on and distributed by:



