Subjects: SVY, PSF
Health Alert: Coalition Urges Awareness of Acetaminophen Safety When Treating Cold and Flu Symptoms This Year
WASHINGTON, Dec. 11, 2018 /PRNewswire/ -- On the heels of the deadliest flu season in 40 years, the Acetaminophen Awareness Coalition (AAC) is urging consumers to double check their medicine labels when treating cold and flu symptoms to avoid doubling up on acetaminophen. While acetaminophen misuse has continued to decline nationwide, accidental overdoses are still more likely to happen during cold and flu season. Research published earlier this year shows that the odds of consumers taking more than the FDA-recommended maximum dose of 4,000 milligrams (mg) of acetaminophen in one day increased 24 percent during cold and flu season. Primarily, that's because more people are using over-the-counter (OTC) combination medications to treat upper respiratory cold and flu symptoms.
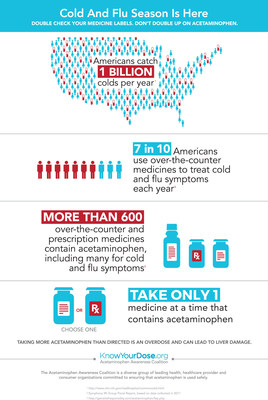
Acetaminophen is safe and effective when used as directed, but there is a limit to how much can be taken in one day. Taking more than directed is an overdose and can lead to liver damage. The U.S. Food and Drug Administration (FDA) has set a maximum daily dose of 4,000 mg of acetaminophen in a 24-hour period.
"Acetaminophen is effective in relieving pain and reducing fever," said Douglas Throckmorton, M.D., deputy director, Center for Drug Evaluation and Research, FDA. "People who are fighting cold and flu viruses should carefully read their medicine labels to avoid taking multiple medications that contain acetaminophen."
The Coalition advises cold and flu sufferers to follow four key acetaminophen safe use steps:
- Always read and follow the medicine label.
- Check the labels on all of your medicines for acetaminophen, which is listed on the front panel of packaging and will be in bold type or highlighted in the "active ingredients" section of OTC medicine labels, and sometimes listed as "APAP" or "acetam" on prescription labels.
- Take only one medicine at a time that contains acetaminophen.
- Ask your healthcare provider if you have questions about dosing instructions or medicines that contain acetaminophen.
The Acetaminophen Awareness Coalition, advised by the FDA, is a diverse group of leading health, healthcare provider, and consumer organizations formed in 2010. The AAC developed the Know Your Dose campaign to educate consumers about safe acetaminophen use in order to prevent liver damage. For more information, visit https://www.knowyourdose.org and follow @KnowYourDose on Twitter.
SOURCE Acetaminophen Awareness Coalition
These press releases may also interest you
|
News published on and distributed by:



