Subjects: PDT, TRI, FDA
ICS 2018: Atlantic Therapeutics Exhibit New Data Demonstrating Comparable Efficacy, Increased Tolerability and Reduced Infection Risk of its Non-invasive Device Innovo® in Treating Stress Urinary Incontinence
PHILADELPHIA, Aug. 28, 2018 /PRNewswire/ -- Atlantic Therapeutics, a global manufacturer of innovative, garment-based pelvic floor muscle strengthening and nerve stimulation products, exhibits data highlighting comparable safety and efficacy of its INNOVO® therapy, an externally worn electrical muscle stimulation device for the treatment of stress urinary incontinence, with greatly reduced risk of infection and improved user tolerability over existing intravaginal probe devices1.
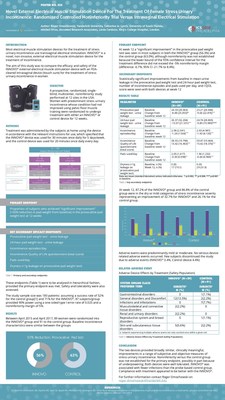
Presented as a poster at the 48th Annual Meeting of the International Continence Society, the data was the result of an FDA designed clinical trial, with patients whose condition had not improved using pelvic floor muscle training. Supporting Atlantic Therapeutics' FDA approval via the De Novo route for INNOVO®. Designed for at home use and worn as a close-fitting pair of shorts, INNOVO® was compared with an existing FDA approved intravaginal stimulation product as control.
At week twelve, 87.2% of the INNOVO® group and 86.8% of the control group were in the dry or mild categories of stress incontinence severity representing an improvement of 32.7% for INNOVO® and 26.1% for the control group1.
Importantly, INNOVO® was associated with fewer infections (0%) than the intravaginal probe control group (7.7%). In wider studies of intravaginal devices, infection rates were reported as high as 14%3.
Compliance with treatment also appeared to be better with INNOVO®1.
A quarter to a third of adults in the U.S. suffer from urinary incontinence2. Standard treatment options begin with physical therapy (PT). In any patient where PT is not successful, pelvic floor stimulation devices may be used, before escalation to the use of injectable bulking agents or surgical interventions involving an implanted mesh sling. Despite this, studies have shown 77% of people have never heard of, or tried, External Electrical Stimulation Devices3.
Significant improvement in stress urinary incontinence is associated with the use of pelvic floor stimulation devices4. Well tolerated and relatively risk-free devices offer advantages and options to patients failing PT, and in a significant group could delay or prevent the need for higher risk surgery or medical intervention.
This latest data shows there is a non-invasive option in INNOVO® that is as effective but far less likely to cause infection and better tolerated than invasive probe-based devices.
References
- ICS 2018, POSTER NO. 235, Roger Dmochowski, Vanderbilt University, Catherine M. Lynch, University of South Florida, Mitchell Efros, AccuMed Research Associates, Linda Cardozo, King's College Hospital, London.
- The Urology Care Foundation (2018) Available at: [http://www.urologyhealth.org/] (Accessed: 24th August 2018).
- Market research undertaken by Tefen on behalf of Atlantic Therapeutics.
- Sand, P. K., D. A. Richardson, D. R. Staskin, S. E. Swift, R. A. Appell, K. E. Whitmore and D. R. Ostergard (1995). "Pelvic floor electrical stimulation in the treatment of genuine stress incontinence: a multicenter, placebo-controlled trial." Am J Obstet Gynecol 173(1): 72-79.
SOURCE Atlantic Therapeutics
These press releases may also interest you
|
News published on and distributed by:



