Subjects: WOM, TRI, FDA
Nestlé Skin Health Announces the FDA Approval of Restylane® Lyft for Hands, the First and Only Hyaluronic Acid (HA) Dermal Filler for Use in the Back of Hands
FORT WORTH, Texas, May 21, 2018 /PRNewswire/ -- Nestlé Skin Health, a global leader focused on meeting the world's increasing skin health needs, announced today that the U.S. Food and Drug Administration (FDA) has approved the hyaluronic acid (HA) dermal filler Restylane® Lyft for the correction of age-related volume loss in the back of the hands for patients over the age of 21. Restylane Lyft is the first and only HA injectable gel to be FDA-approved for restoring fullness to the back of the hands2,3, providing more youthful-looking skin.4 It is also the first-ever HA dermal filler to receive FDA approval for an area other than the face."2,3
?The Restylane Survey was conducted among 1,000 nationally representative U.S. women, ages 35+, between February 26 and March 5, 2018, using an email invitation and an online survey.
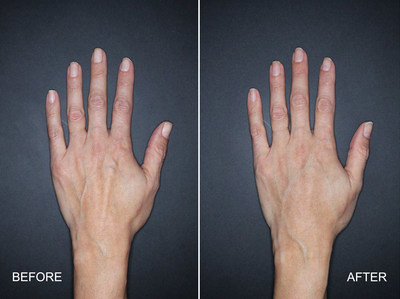
"As we age, we lose volume in the back of the hands, causing the appearance of tendons, wrinkles and veins to become more pronounced,"5 stated Dr. Ellen Marmur, a board-certified dermatologist in New York City and clinical trial investigator for Restylane Lyft in the hands. "Many of my patients start to notice their youthful-looking faces aren't matching their aging hands, and ask me what they can do about it. I am now pleased to be able to offer them Restylane Lyft, an injectable HA treatment that many are familiar with since it has been on the market for over 10 years for facial wrinkles."6
"This new indication demonstrates Nestle Skin Health's mission to deliver a stream of innovative aesthetic solutions that help bring new patients into the market," said Alisa Lask, General Manager and Vice President, U.S. Aesthetic Business Unit at Nestlé Skin Health. "Restylane Lyft is now the first and only dermal filler with 3 FDA-approved treatment areas ? the midface/cheek area, nasolabial folds, and now the back of the hands."2,3
This FDA approval was based on a multi-center, randomized, evaluator-blinded, split-hand study investigating safety and efficacy of Restylane Lyft for use in the dorsal (back side) of the hands. Eighty nine patients age 22 and over were enrolled in the trial. The study met its primary endpoint, showing a clinically meaningful improvement in the correction of volume deficits of treated hands for up to six months. Restylane Lyft was shown to be both safe and well-tolerated, for the correction of volume deficit in the dorsal (back side) hand. After initial treatment, injection site responses (swelling, tenderness, redness, bruising, pain, itching, impaired hand function) were predominantly mild in intensity, and temporary.4
"As demonstrated in this first-in-class clinical trial, Restylane Lyft offers a safe and effective HA treatment option for restoring volume to the hands and delivering natural-looking results," added Dr. David Bank, New York dermatologist and clinical investigator for Restylane Lyft in the hands. "This new treatment allows me to address the 65% of women over the age of 35 that believe their hands make them look older than their age." 1?
Hyaluronic acid is a natural occurring substance in the skin that helps provide fullness and elasticity. It diminishes as we age, and causes the skin to lose volume, while increasing the chances for wrinkles to appear.
With over 30 million treatments worldwide and counting7, the Restylane® family of HA dermal fillers is the broadest portfolio of dermal fillers in the U.S. The portfolio of products help to smooth facial wrinkles and folds, such as smile lines (Restylane®-L, Restylane® Refyne, Restylane® Defyne and Restylane® Lyft with Lidocaine), create fuller and more accentuated lips (Restylane® Silk and Restylane®-L), and add lift and volume to the cheeks and the back of the hands (Restylane® Lyft with Lidocaine).
To learn more about the Restylane family of products, visit www.RestylaneUSA.com.
Nestlé Skin Health's mission is to enhance quality of life by delivering science-based solutions for the health of skin, hair and nails. As one of the category's leading companies, Nestlé Skin Health conducts ground-breaking product research to provide both the healthcare community and the consumer with an ongoing progression of innovative technologies and products to protect, serve and enhance skin health. For more information, please visit www.nestleskinhealth.com.
To earn exclusive rewards, bonuses and discounts on Nestlé Skin Health's aesthetic treatments, join the ASPIRE Rewards program. To learn more about ASPIRE, visit www.aspirerewards.com.
Important Safety Information
The Restylane family of products includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Refyne, and Restylane® Defyne.
APPROVED USES
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips in patients over the age of 21.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies in patients over the age of 21. Restylane® Lyft with Lidocaine is also indicated for injection into the subcutaneous plane in the dorsal hand to correct volume deficit in patients over the age of 21.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles in patients over the age of 21.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds, in patients over the age of 21.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds, in patients over the age of 21.
Are there any reasons why I should not use products within the Restylane® family? (Contraindications)
To ensure a safe procedure, your doctor will talk to you about your medical history to determine if you are an appropriate candidate for treatment. You should not use products within the Restylane family if:
- You have severe allergies with a history of severe reactions (anaphylaxis)
- You are allergic to lidocaine or to any of the gram-positive bacterial proteins used to make hyaluronic acid
- You are prone to bleeding or have been diagnosed with a bleeding disorder
Are there other precautions that I should discuss with my doctor?
- Tell your doctor if you are breastfeeding, pregnant, or trying to become pregnant. The safety of these products for use during pregnancy, or in women who are breastfeeding, has not been studied
- Restylane, Restylane-L, Restylane® Lyft with Lidocaine, Restylane Refyne and Restylane Defyne are intended to treat facial wrinkles and folds, such as nasolabial folds. Restylane and Restylane-L are also intended for lip enhancement. Restylane® Lyft with Lidocaine is also intended for injection in the dorsal hand to correct volume loss. Treatments in other areas of the face or body have not been evaluated in clinical studies.
- The safety and effectiveness of Restylane® Silk for areas other than the lips and perioral area have not been evaluated in clinical studies.
- Tell your doctor if you have any history of scarring, particularly thick and stiff scars, or any pigmentation (skin color) disorders. These side effects can occur with hyaluronic acid fillers in general.
- Tell your doctor if you are planning other laser treatments or a chemical peel, as there is a possible risk of inflammation at the treatment site if these procedures are performed after treatment
- Patients who experience skin injury near the site of injection with these products may be at a higher risk for side effects
- Tell your doctor if you are on any medications to decrease your body's immune response (immunosuppressive therapy). Using these medications may increase your risk of bruising or bleeding at the gel injection site.
- Tell your doctor if you are using any "blood thinners" such as aspirin, warfarin, or any other medications that affect bleeding. Using these medications may increase your risk of bruising or bleeding at the gel injection site.
- The use of these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete. Use of product in these areas could delay healing or make your skin problems worse.
Tell your doctor if you have diseases, injuries, or disabilities of the hand.
What are the possible side effects?
The most commonly observed side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. These are typically mild in severity and typically resolve in less than 7 days in nasolabial folds and less than 14 days in lips. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site.
One of the risks with using this product is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious, and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin.
As with all skin injection procedures, there is a risk of infection.
To report a side effect with any of the Restylane products, please call Galderma Laboratories, L.P at 1-855-425-8722.
The Restylane family of products is available only through a licensed practitioner. Complete Instructions for Use are available at www.RestylaneUSA.com.
1 Data on file. Restylane Survey Demographic Report (Wakefield Research), March 2018.
2 U.S. Food & Drug Administration. Dermal Fillers Approved by the Center for Devices and Radiological Health. Accessed May 15, 2018. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm.
3 Restylane Lyft. Instructions for Use. Fort Worth, TX. Galderma Laboratories, L.P., 2018
4 Data on file. 43USH1501 Tables, Figures, Listings. Fort Worth, TX: Galderma Laboratories, L.P., 2018.
5 Fabi SG, Goldman MP. Hand rejuvenation: a review and our experience. Dermatol Surg. 2012 Jul;38(7 Pt 2):1112-27. doi: 10.1111/j.1524-4725.2011.02291.x.
6 PERLANE® Injectable Gel. Summary of Safety and Effectiveness Data (PMA P040024/S6). 2007:1-16.
7 Data on file. MA-31037 document. Fort Worth, TX: Galderma Laboratories, L.P., 2016.
© 2018 Galderma Laboratories, L.P.
All trademarks are the property of their respective owners.

SOURCE Nestlé Skin Health
These press releases may also interest you
|
News published on and distributed by:



