Subject: LIC
Delta Development Team Receives SBIR Phase Two Enhancement for Innovations in Battlefield Blood Storage
TUCSON, Ariz., March 28, 2024 /PRNewswire/ -- Delta Development Team (DDT), a leader in medical device innovation, has been awarded a Small Business Innovation Research (SBIR) phase two enhancement from the Defense Health Agency (DHA) for its groundbreaking advancements in cold chain logistics systems for far-forward blood delivery, specifically tailored for point-of-injury care on the battlefield. This accolade underscores DDT's commitment to revolutionizing medical logistics for military operations.
For the past five years, DDT has been diligently working on this system, marking it as their third FDA-listed medical device for blood delivery. Their innovative approach involves designing autonomous devices that work seamlessly with military-standard batteries for extended periods, ensuring continuous operation in the most challenging environments.
The APRU and Delta Ice are two other robust medical devices developed by DDT that have proven their effectiveness in the field, saving lives in both Ukraine and Israel, as well as all over the world in both military and EMS applications. These devices are being utilized in different ways to transport blood to the point of injury. In Ukraine, the APRU is employed for stabilization during damage control resuscitation, while the Delta Ice is used for drone blood resupply. In Israel, they are a crucial part of the special operations forces and are being used in armored vehicles as part of mobile damage control teams. These devices have been instrumental in providing critical medical support in high-pressure situations. They also have been used by EMS to save lives when getting to a trauma center is challenging or a great distance.
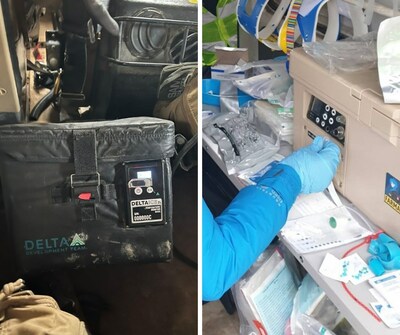
The flagship product in DDT's arsenal is the Autonomous Portable Refrigeration Unit (APRU), globally recognized for its ability to store blood in extreme conditions ranging from 50°C down to -32°C. Equipped with specialized software, the APRU efficiently manages power consumption and performs optimally even in the harshest temperatures. Notably, it features a warming element to prevent blood from freezing during arctic operations, ensuring the integrity of vital medical supplies.
In addition to the APRU, DDT has introduced a smart blood cooler tailored for drone usage and short-duration missions from forward operating bases. This cooler boasts a countdown and alert system, notifying personnel when it's time to replace the temperature control insert, thereby guaranteeing continuous and safe blood storage during critical missions.
Furthermore, DDT has developed the Transportable Blood Storage (TBS), a 10-day powered refrigerator designed to increase blood supply capacity on the battlefield. Its compact size and weight render it suitable for mobile applications in various military vehicles and trucks. Notably, the TBS is engineered for easy maintenance and repair in the field, with cannibalizable parts that allow for quick fixes by general technicians, eliminating the need for highly specialized personnel.
The overarching goal of DDT's project is to deliver a maintainable, scalable, and sustainable system that is adaptable to field or theater operations. Overcoming the challenge of maintaining the cold chain while ensuring timely availability of blood at the point of injury is central to their mission. This system of systems is poised to revolutionize medical logistics across all military medical facilities, offering the necessary capacity and durability for seamless integration into military operations.
They are proud to have achieved another SBIR (previously awarded the AFWERX Phase I and II) to continue to develop life-saving technology that they are passionate about.
Contact: [email protected]
SOURCE Delta Development Team
These press releases may also interest you
|
News published on and distributed by: