Subject: PDT
PROTEONIC UNVEILS EARLY ACCESS PROGRAM, ELEVATING VIRAL VECTOR TITERS WITH TRANSFORMATIVE LV-2G UNIC® TECHNOLOGY
LEIDEN, Netherlands, March 27, 2024 /PRNewswire/ -- ProteoNic Biosciences, a leading provider of premium vector technology and services for the production of biologics, is announcing the launch of its highly anticipated LV-2G UNic® Early Access Program. This program marks a significant milestone in lentivirus manufacturing optimization, offering access to groundbreaking vector technology designed to revolutionize viral vector production.
With the official launch on March 20, 2024, ProteoNic extends a warm invitation to CDMOs, biotechs, and biopharmaceutical companies to experience firsthand the transformative potential of LV-2G UNic®. By seamlessly integrating this cutting-edge vector technology into existing systems, participants can achieve remarkable enhancements in titers, paving the way for increased viral vector production capacity and substantial improvements in manufacturing cost efficiency.
Frank Pieper, CEO of ProteoNic, shared his excitement about the program's potential impact, stating, "The LV-2G UNic® Early Access Program will serve as a launching platform for our viral vector manufacturing innovation. By offering early access to our state-of-the-art vector technology, we empower researchers to unlock unprecedented levels of efficiency and productivity in viral vector manufacturing."
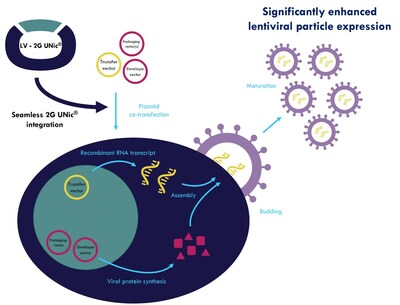
LV-2G UNic® represents next generation vector technology, engineered to elevate lentiviral titers across a wide array of expression systems. The technology drives enhanced infectious viral particle production, by providing a significant boost in gRNA production through increased transcription levels achieved with ProteoNic's patented Dual Promoter system.
Participation in the LV-2G UNic® Early Access Program offers exclusive benefits, including access to ProteoNic's cutting-edge LV-2G UNic® vector technology, optimizing lentiviral particle expression and co-development opportunities. This technology can be seamlessly integrated into current workflows with minimal to no process development required.
For more information about LV-2G UNic® and the Early Access Program, visit proteonic.nl
About ProteoNic BV
ProteoNic is a privately held company with offices in Leiden, the Netherlands and in the Boston area, USA. The company offers technology and services for the generation of cell lines and viral vectors with greatly improved production characteristics. The company commercializes its proprietary 2G UNic® technology through licensing and partnership arrangements.
For more information please contact:
ProteoNic BV
Jonathan Frampton, PhD
Director, Business Development
T: +44 7961 144115
E: [email protected]
Photo - https://mma.prnewswire.com/media/2373127/ProteoNic.jpg
Logo - https://mma.prnewswire.com/media/1804728/ProteoNic_Logo.jpg
SOURCE ProteoNic
These press releases may also interest you
|
News published on and distributed by: