Subject: TRI
New ASCCP cervical cancer management guidelines now include dual-stain triage testing with Roche's CINtec® PLUS Cytology to enable earlier diagnosis
- Updated American Society of Colposcopy and Cervical Pathology (ASCCP) guidelines recognize the importance of dual-stain biomarkers in managing early diagnosis of HPV- positive cervical precancer and cancer.
- Earlier detection may significantly reduce the number of women expected to be diagnosed with cervical cancer.
- CINtec® PLUS Cytology is the only FDA-approved dual-stain triage test for HPV-positive cervical cancer screening results.
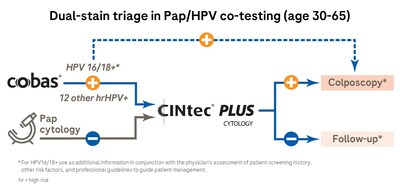
INDIANAPOLIS, March 11, 2024 /PRNewswire/ -- Roche Diagnostics today announced the release of new guidelines from ASCCP and other members of the Enduring Guidelines for Cervical Cancer Screening and Management Committee that now recognize dual-stain biomarkers as an important technology in helping clinicians triage patients to determine if their human papillomavirus (HPV) infection is transforming into cervical pre-cancer. Roche's CINtec® PLUS Cytology is the only FDA-approved dual-stain triage test for HPV-positive cervical cancer screening results.
Dual stain is a robust marker of CIN3+ risk and can be incorporated into clinical management strategies, according to the guidelines.? Existing clinical decision support tools (e.g., the ASCCP app) plan to incorporate these recommendations for use of DS.
The guidelines examine data from the Kaiser Permanente Northern California cohort and the STudying Risk to Improve DisparitiES study, and outline new recommendations on how to manage patients using dual-stain technology. The guidelines also note that compared with cytology, dual stain requires fewer colposcopies and detects cervical intraepithelial neoplasia grade 3 or greater earlier.
"There is robust evidence that proves the use of dual-staining to risk stratify and triage patients is superior to cytology, making it a valuable tool for OB-GYNs," said Dr. Tamera Paczos, an OB-GYN and senior vice president, chief laboratory officer for BioReference, which operates large reference facilities nationwide. "Dual-stain triage provides excellent discrimination between HPV-positive individuals requiring direct colposcopy and those who can be safely followed in one year. It can provide peace of mind to women who are often asked to wait a year until their next screening before knowing whether their HPV infection may resolve on its own, or remain persistent and transform into cancer."
When detected at an early stage, the five-year survival rate for women with invasive cervical cancer is 92%.1 In the IMPACT clinical trial, testing with CINtec PLUS Cytology as a triage detected cervical disease earlier than Pap cytology in seven out of 10 women who were already identified as HPV positive.2,3 The biomarker-based CINtec PLUS Cytology test showed a significantly higher sensitivity in detecting cervical pre-cancers, compared to Pap cytology when used for the triage of HPV-positive women. This allows clinicians to treat disease earlier, a critical factor in improving cancer patient outcomes.
"For the first time, ASCCP has included dual-stain testing as an option for the triage of HPV-positive women in HPV primary screening and co-testing settings," said Laura Shields, chief medical partner, Pathology Oncology at Roche Diagnostics. "With more advanced technologies, such as dual-stain, better access and more awareness, we can change the course of this disease with earlier detection, save lives and eventually eliminate cervical cancer."
CINtec® PLUS Cytology detects the simultaneous presence within a single cell of the p16 and Ki-67 biomarkers, which are associated with HPV infections that are transforming and may lead to cervical cancer. A positive test result signals that a patient has a significantly higher risk for the disease. The dual-stain test is performed with the same sample as used for HPV or Pap cytology tests, so it does not require a repeat office visit for the patient. The Roche cobas® HPV Test is the only test approved by the FDA for use with CINtec PLUS.
?Please see ASCCP website (acssp.org) for full details on the updated guidelines.
About the Roche Cervical Cancer Portfolio
Roche's cervical cancer portfolio includes the cobas HPV Test, used for primary screening and co-testing, which tests for 14 types of high-risk HPV genotypes in cervical cancer. It includes results for HPV 16, HPV 18 and 12 other high-risk pooled genotypes.4 The portfolio also includes CINtec PLUS Cytology, the only FDA-approved dual-stain product and CINtec® Histology, the only FDA-cleared p16 biomarker technology that can help pathologists confirm the presence of precancerous cervical lesions.
The IMPACT trial design, used to validate the clinical benefits of the Roche cervical cancer portfolio, had representation from diverse patient segments, including 21 percent Black, 24 percent Hispanic-Latino and .3 percent American Indian or Alaskan Native participants. This diversity was critical to accurately assess the performance of dual stain in patient populations with higher incident rates of HPV.5
About Roche
Founded in Basel, Switzerland, in 1896 as one of the first industrial manufacturers of branded medicines, Roche has grown into the world's largest biotechnology company and the global leader in in-vitro diagnostics. The company pursues scientific excellence to discover and develop medicines and diagnostics for improving and saving the lives of people around the world. We are a pioneer in personalised healthcare and want to further transform how healthcare is delivered to have an even greater impact. To provide the best care for each person, we partner with many stakeholders and combine our strengths in Diagnostics and Pharma with data insights from the clinical practice.
In recognizing our endeavor to pursue a long-term perspective in all we do, Roche has been named one of the most sustainable companies in the pharmaceuticals industry by the Dow Jones Sustainability Indices for the thirteenth consecutive year. This distinction also reflects our efforts to improve access to healthcare together with local partners in every country we work.
Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan.
For more information, please visit www.roche.com.
All trademarks used or mentioned in this release are protected by law.
Roche Diagnostics U.S. Media Relations
Krystina Monaco
(317) 850-7521
[email protected]
Lori McLaughlin
(463) 207-2395
[email protected]
[email protected]
1 | Cancer.net editorial board, Jan. 1, 2022. https://www.cancer.net/cancer-types/cervical-cancer/statistics#:~:text=When%20detected%20at%20an%20early,year%20survival%20rate%20is%2058%25. |
2 | CINtec® PLUS Cytology. Package insert. Roche Diagnostics; 2020. |
3 | Safaeian M, Wright TC Jr, Stoler MH, Ranger-Moore J, Rehm S, Aslam S, Fang Q, Volkir P, Ridder R. The IMproving Primary Screening And Colposcopy Triage trial: human papillomavirus, cervical cytology, and histopathologic results from the baseline and 1-year follow-up phase. Am J Obstet Gynecol. 2021 Sep;225(3):278.e1-278.e16. doi: 10.1016/j.ajog.2021.03.047. Epub 2021 Apr 20. |
4 | cobas® HPV test [package insert]. Branchburg, NJ: Roche Molecular Systems, Inc.; 2020. |
5 | Wright TC Jr, Stoler MH, Ranger-Moore J, Fang Q, Volkir P, Safaeian M, Ridder R. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: Results from the IMPACT trial.Int J Cancer. 2022 Feb 1;150(3):461-471. doi: 10.1002/ijc.33812. Epub 2021 Sep 25 |
SOURCE Roche Diagnostics
These press releases may also interest you
|
News published on and distributed by: