Subjects: PSF, CFG
Public Advisory - Unauthorized health products seized from Tokyo Beauty and Healthcare store in Richmond, B.C., may pose serious health risks
OTTAWA, ON, March 8, 2024 /CNW/ - UPDATE: March 8, 2024: Additional unauthorized health products seized from another Tokyo Beauty and Healthcare store in Richmond, B.C.
Further to its communication on September 18, 2023, Health Canada is warning consumers about additional unauthorized health products seized from another Tokyo Beauty and Healthcare store located at Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. These products are labelled to contain prescription, controlled, or over-the-counter drugs, and may pose serious health risks.
The list of products seized has been added to the Affected Products table below. Information on the health risks associated with these drugs as well as information for consumers can be found further below.
Original Advisory: September 18, 2023: Unauthorized health products seized from a Tokyo Beauty and Healthcare store in Richmond, B.C., may pose serious health risks
Product: Unauthorized health products labelled to contain prescription, controlled or over-the-counter drugs
Issue: Health products - Product safety
What to do: Do not use these products. Buy your prescription drugs only from licensed pharmacies. Return products to your local pharmacy for proper disposal. Consult a health care professional if you have used any of these products and have health concerns. One of the products (Pabron Gold A Granules Cold Medication) contains an opioid. If you suspect an opioid overdose, call 911 and administer naloxone if available.
Health Canada is warning consumers about unauthorized health products it seized from a Tokyo Beauty and Healthcare store in Richmond, B.C. (120 - 8191 Westminster Highway). The products are labelled to contain prescription, controlled or over-the-counter drugs and may pose serious health risks.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, efficacy and quality and may pose a range of serious health risks. For example, they could contain high-risk ingredients, such as prescription drugs, additives or contaminants that may or may not be listed on the label. These ingredients could interact with other medications and foods. In addition, these products may not actually contain the active ingredients that consumers would expect them to contain to help maintain and improve their health.
Prescription drugs should only be used under the advice and supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects. Prescription drugs can only be legally sold to consumers in Canada with a prescription.
Product | Promoted use | Drug on the label | Tokyo Beauty and Healthcare store |
Pabron Gold A Granules Cold Medication | Relieve cold symptoms | Labelled to contain dihydrocodeine phosphate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Kobayashi Pharmaceutical Faichi Iron + Folic Acid + Vitamin B12 Blood Supplement Tablets | Aid production of blood hemoglobin and improve anemia | Labelled to contain prescription-strength folic acid | 120 - 8191 Westminster Highway, Richmond, B.C. |
Mentholatum Mediquick Eczema Rash Anti-Itch Cream | Eczema and rash relief | Labelled to contain prednisolone valerate acetate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Mentholatum Mediquick Eczema Rash Anti-Itch Ointment | Eczema and rash relief | Labelled to contain prednisolone valerate acetate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Nichiban Speel Ko One-touch EX | Corns, callus and wart removal | Labelled to contain salicylic acid | 120 - 8191 Westminster Highway, Richmond, B.C. |
Ohta's Isan A | Aid digestion and relieve heartburn | Labelled to contain ursodeoxycholic acid | 120 - 8191 Westminster Highway, Richmond, B.C. |
Santen Beauteye Contact | Eye fatigue | Labelled to contain neostigmine methylsulfate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Santen PC Eyedrops | Eye inflammation and fatigue | Labelled to contain neostigmine methylsulfate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Smile 40EX Gold Cool MAX | Eye fatigue, blurred vision | Labelled to contain neostigmine methylsulfate | 120 - 8191 Westminster Highway, Richmond, B.C. |
Santen Sante Beauteye Moon Care | Prevention of eye disease, conjunctival congestion, bleary eyes | Labelled to contain aminocaproic acid | Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
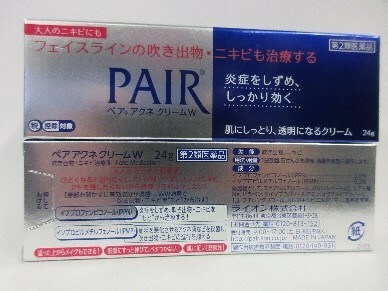
Pair Acne Cream | Acne skin treatment | Labelled to contain ibuprofen piconol 3% | Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Pabron Gold A Granules Cold Medication | Relieve cold symptoms | Labelled to contain dihydrocodeine phosphate | Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Mentholatum Mediquick Eczema Rash Anti-Itch Ointment | Eczema and rash relief | Labelled to contain prednisolone valerate acetate | Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
Hadalabo Pearl Barley Face Wash | Skin treatment | Labelled to contain aminocaproic acid | Unit 3660 - 4151 Hazelbridge Way, Richmond, B.C. |
- Do not use these products. Return the product to your local pharmacy for proper disposal.
- Consult a health care professional if you have used any of these products and have health concerns.
- Pabron Gold A Granules Cold Medication contains an opioid. Opioid overdose is a medical emergency that could lead to death if untreated. If you suspect an opioid overdose, call 911 and administer naloxone if available.
- Buy your prescription drugs only from licensed pharmacies.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related side effects or complaints to Health Canada.
Aminocaproic acid is a prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Dihydrocodeine phosphate is a controlled substance regulated under the Controlled Drugs and Substances Act and is similar to the opioid codeine. Although codeine is approved in Canada, Health Canada has not authorized any drug products containing dihydrocodeine. Dihydrocodeine tablets are approved in certain countries for the relief of severe and chronic pain or as cough suppressants. Common adverse reactions include dizziness, headache, vertigo, visual disturbances, confusion, euphoria, nausea, and constipation. As with all opioids, use of dihydrocodeine may lead to drug dependence (addiction). Dihydrocodeine may cause slowed or stopped breathing, which can lead to severe drowsiness, unconsciousness, and death (opioid overdose). People with other medical conditions, including but not limited to those that affect the lungs, liver or kidney, may be at a higher risk of overdose. Children are more susceptible to overdose due to their smaller size. Dihydrocodeine should not be used by those who are pregnant or breastfeeding, or by those with an allergy to dihydrocodeine. Using dihydrocodeine with other central nervous system depressants (e.g. alcohol, sleeping pills, etc.) may worsen its effects. In cases of overdose, naloxone can temporarily reverse the effects of dihydrocodeine.
Folic acid tablets at strengths above 1 mg are prescription drugs available in Canada for the prevention and treatment of: folate deficiency, birth defects, and side effects related to methotrexate treatment. Folic acid should not be taken at doses of more than 1 mg per day without the advice of a health care professional. Taking too much folic acid may hide signs of a vitamin B12 deficiency and increase the risk of progressive, unrecognized neurological damage. Taking too much folic acid may also increase the risk of colorectal and possibly other cancers in certain individuals. High doses of folic acid might lead to symptoms, including loss of appetite, nausea, abdominal bloating, gas, bitter taste, changes to sleep, trouble concentrating, depression, impaired judgement, and feeling irritable, excited, overactive or confused. Rare but serious side effects include severe allergic reactions. Folic acid should not be used by people who have untreated vitamin B12 deficiency. Oral folic acid is not to be used by people who have diseases of the small intestine, especially Crohn's disease and celiac disease, due to difficulties absorbing folic acid.
Ibuprofen piconol 3% is a topical (applied to the skin) non-steroidal anti-inflammatory drug (NSAID) used to relieve burns. Health Canada has not approved any drugs containing ibuprofen for topical use. Ibuprofen absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time, or on damaged skin. Topical ibuprofen may cause serious side effects in people who are allergic to ibuprofen, aspirin or other NSAIDs, or who are asthmatic. Use of topical ibuprofen may also cause serious side effects such as stomach and intestinal bleeding, renal (kidney) dysfunction or failure, or cardiovascular dysfunction or failure in people who have problems with these organs. Topical ibuprofen can also cause serious side effects in people who are pregnant or breastfeeding, such as delayed and increased duration of labour.
Neostigmine methylsulfate is a prescription drug available in Canada as an injection used to prevent and treat urine retention and intestinal complications after surgery, to reverse the paralyzing effect of certain drugs used in surgery and in shock therapy, and to control symptoms of myasthenia gravis (a disease that causes weakness in the skeletal muscles). It has not been approved for use as eye drops in Canada. In the past, drugs similar to neostigmine were used to treat glaucoma, a group of eye diseases traditionally characterized by elevated pressure within the eye. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts (clouding of the normally clear lens of the eye), allergic reactions, iris cysts, retinal detachment with symptoms of reduced vision, sudden appearance of flashes of light or floaters that could lead to permanent vision loss, and the potential for causing a specific type of glaucoma attack with potential permanent vision loss. In addition, absorption into the nose via the tear duct may cause serious cardiac and respiratory side effects.
Prednisolone valerate acetate is a prescription corticosteroid drug available in Canada as eye drops used to treat inflammation of several parts of the eye. It has not been approved for use in creams or ointments in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), change in skin color, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, and swelling. Prednisolone acetate should not be used in patients who are allergic to prednisolone acetate or to any ingredient in the formulation. Prednisolone acetate is not to be used in children and is not recommended for use during pregnancy or breastfeeding.
Salicylic acid is a prescription drug when sold for topical uses at concentrations greater than 20% or with a certain level of acidity (pH less than 3.0) except when sold to be applied to warts, corns or calluses. It is also used to treat acne. It should not be used by people who are allergic to salicylic acid, by people with diabetes, poor circulation, loss of sensation in the extremities (e.g., hands and feet), or by children and teenagers with the flu or chicken pox (as it may increase the risk of Reye's syndrome, a serious disease that might lead to seizures and coma). It should not be used by pregnant or nursing people unless the area of exposure and duration of therapy is limited. Some products containing salicylic acid should not be used in children younger than two years of age. Salicylic acid can cause serious allergic reactions (hives, itching, trouble breathing, and swelling of the face, lips, or tongue), and severe skin irritation (redness, burning, dryness, itching, and peeling). It can also cause salicylate toxicity, a serious condition with nausea, vomiting, dizziness, loss of hearing, ringing in the ears, diarrhea, confusion, rapid breathing, and drowsiness. Side effects are more likely to occur in children and in people with kidney or liver disease, and with prolonged use over large areas. Salicylic acid should not be used on infected areas, on moles, birthmarks, warts with hair growing from them, or warts on the face.
Ursodeoxycholic acid is a prescription drug used for the management of cholestatic liver diseases (diseases that involve blocked or reduced bile flow from the liver). Serious side effects of taking ursodeoxycholic acid include allergic reactions, chest pain and difficulty breathing, stomach ache, nausea, diarrhea or constipation, swelling of the extremities (e.g., hands and feet), high blood pressure, fatigue, dizziness, headache, itchiness, fever and jaundice. Some patients have experienced additional symptoms such as vomiting and pain in the abdominal area caused by blockages in the gastrointestinal tract, which requires medical intervention. Blood tests are needed to monitor for the risk of liver toxicity from taking this drug. Ursodeoxycholic acid should not be used by people who have an allergy to ursodiol, have a blockage of bile flow due to liver or other disease, or by people who are pregnant, planning to become pregnant, or breastfeeding.
Également disponible en français
SOURCE Health Canada (HC)
These press releases may also interest you
|
News published on and distributed by: