Subject: FDA
Tyber Medical Acquires FDA Clearance for Proximal Tibia Plating System
Featuring Single/Multiple Component Metallic Bone Fixation
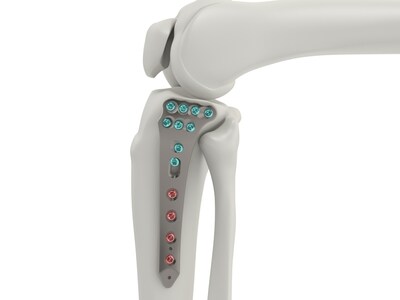
BETHLEHEM, Pa., Feb. 12, 2024 /PRNewswire/ -- Tyber Medical LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, has received a U.S. Food and Drug Administration (FDA) 510(k) approval for its Proximal Tibia Plating System. The Tyber Medical System consists of two indication categories: Complete Articular and Partial Articular Fractures. Both designs stabilize bone fragments to facilitate highly focused and efficient healing of a wide array of injuries to the tibia, fibula, and femur. "This innovative addition to the Tyber Medical portfolio aligns to meet the needs of our customers by offering multiple solutions to challenging anatomy," commented David Hannah, Chief Technology Officer at Tyber Medical. Mr. Hannah added. "This comprehensive system offers anatomic plating to support a wide range of trauma needs."
The Tyber Medical Proximal Tibial Plating System is engineered to accommodate surgeon preferences, increase procedural efficiency, and enhance plate fit and screw placement. The Proximal Tibia system is anatomically designed to address a variety of indications and includes variable angle locking and non-locking screws. Lisa Boyle, Director of Regulatory Affairs commented "With the clearance of the Proximal Tibial Plating System and its sub-categories, Tyber Medical remains unwavering in our commitment to providing cutting edge orthopedic solutions."
About Tyber Medical LLC
Tyber Medical LLC, is a leading orthopedic device manufacturer providing rapid access to FDA-cleared and CE-marked, portfolio-enhancing, regulatory-approved orthopedic implants for customer private labeling in the spinal, extremity, and trauma markets. Tyber Medical provides customers with a quick and seamless path to market. Since it was founded in 2012, the company has released more than 50 spine, extremity, and trauma systems. Tyber Medical aims to develop and utilize differentiated technologies to make advanced orthopedic implants.
Contact: Eric Dickson, Tyber Medical
Phone: 610-849-1710
Email: [email protected]
SOURCE Tyber Medical
These press releases may also interest you
|
News published on and distributed by: