Subject: FDA
Tyber Medical Anatomical Plating System Cleared For Canada
Comprehensive portfolio of fixation devices for the extremities is now available
BETHLEHEM, Pa., Feb. 6, 2024 /PRNewswire/ -- Tyber Medical LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, received clearance for the anatomical plating system in Canada. The comprehensive portfolio previously received FDA 510(k) in the US and has now been cleared through Health Canada.
Tyber Medical's CEO and President, Jeff Tyber, announced that the company has received clearance from Health Canada to offer a comprehensive plating solution for long and short bone trauma and deformity. "We are excited about this development and look forward to expanding our presence in the orthopedic plating arena," said Tyber.
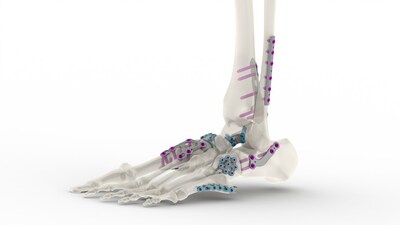
The plating system incorporates a series of stainless steel and titanium plates that incorporate standard and variable angle locking compression plates and screws of varying lengths, thicknesses, and configurations. The low-profile plates minimize soft tissue irritation, while maximizing the variable locking technology to reduce screw head prominence. Additionally, the sterile and non-sterile plates include an engineered combination of material and surface modification that significantly increases fatigue resistance.
Chief Commercial Officer Kevin Brothen said, "This additional clearance for Canada further cements Tyber Medical as a complete global source for orthopedic plating and we are excited for the future."
Tyber Medical is committed to developing rapid access, innovative, orthopedic device technology to advance patient care and healing outcomes. The company's extensive medical device portfolio effectively supports gaps in its partners' product offerings in as little as four months through vertically integrated design, engineering, manufacturing, quality management, regulatory, clinical research, and MDR compliance.
About Tyber Medical LLC
Tyber Medical LLC, is a leading orthopedic device manufacturer providing rapid access to FDA-cleared and CE-marked private label, portfolio-enhancing, regulatory-approved orthopedic implants for the spinal interbody space and extremity/trauma markets. Tyber Medical provides customers with a quick and seamless path to market. Since it was founded in 2012, the company has released more than 50 extremity/trauma/spine systems. Tyber Medical aims to develop and utilize differentiated and bioengineered technologies, including different surface treatments and coatings, to make advanced orthopedic implants.
Contact: Eric Dickson, Tyber Medical
Phone: 610-849-1710
Email: [email protected]
SOURCE Tyber Medical
These press releases may also interest you
|
News published on and distributed by: