Subjects: PDT, FDA
U.S. FDA Approves the VISUMAX 800 with SMILE pro software from ZEISS
The updated ZEISS femtosecond laser provides U.S. refractive surgeons with faster treatment, greater flexibility, and significant workflow enhancements.
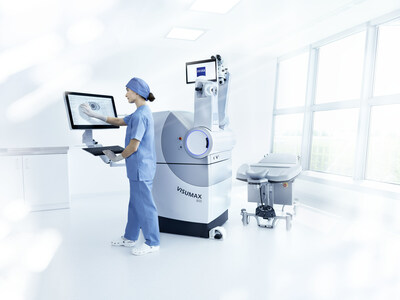
DUBLIN, Calif. and JENA, Germany, Jan. 11, 2024 /CNW/ -- Carl Zeiss Meditec AG -- ZEISS Medical Technology announced today that the U.S. Food and Drug Administration (FDA) has approved the VISUMAX® 800 with SMILE® pro software from ZEISS for surgically treating nearsightedness, with or without astigmatism. The latest generation of femtosecond lasers from ZEISS enters the U.S. market at a time when more than 8 million eyes have been treated with SMILE worldwide, reflecting the technology's broad global momentum driven by strong adoption in Asia and Europe.
"The increasing global adoption of SMILE from ZEISS represents the positive impact the small incision lenticule extraction procedure continues to have on the quality of life for patients," said Andrew Chang, Head of Global Sales for ZEISS Medical Technology. "With the availability of the ZEISS SMILE pro software module in the U.S. market, surgeons can now offer the latest refractive technology to help expand their business and provide excellent outcomes for patients."
"ZEISS continues to set itself apart in the U.S. market with the availability of the VISUMAX 800 with SMILE pro software from ZEISS, providing the latest digital technology from the company's legacy of innovation to meet the evolving needs of refractive surgeons," said Euan S. Thomson, Ph.D., President of Ophthalmology Strategic Business Unit and Head of the Digital Business Unit for ZEISS Medical Technology. "As part of the ZEISS Medical Ecosystem, this next generation femtosecond laser system creates data-driven insights to help surgeons manage better treatment paths for patients while supporting each surgeon's unique practice requirements for greater workflow efficiency and performance."
The VISUMAX® 800 with SMILE® pro software from ZEISS enables faster treatment, creating the lenticule in less than 10 seconds thanks to a faster laser pulse repetition rate of 2 MHz.* Additionally, the shorter procedure time can reduce stress for surgeons and their patients. The ZEISS femtosecond laser also provides greater flexibility for the surgeon and patient, with a smaller footprint and compatibility with a variety of patient beds, adapting to the clinical environment to provide cutting-edge technology without compromise.
With the availability of the VISUMAX® 800 with SMILE® pro software from ZEISS in the U.S., surgeons can utilize a number of workflow enhancements including: the CentraLign® centration aid, a computer-controlled function for easy centration; the OcuLign® cyclotorsion adjustment to help counter cyclotorsion that may occur; and VISULYZE user nomograms to help surgeons collect and analyze patient data, while also providing detailed nomograms and enabling more control during every surgery.
With these workflow enhancements along with greater flexibility and faster treatment, the VISUMAX® 800 with SMILE® pro software from ZEISS offers substantial market opportunities for U.S. surgeons. "The VISUMAX 800 is a practice builder and allows surgeons to differentiate their practice from local competitors," said Luke Rebenitsch, MD, at ClearSight LASIK and 43Vision, in Oklahoma City, Oklahoma, USA.
For more information about the VISUMAX® 800 with SMILE® pro software from ZEISS, visit www.zeiss.com/us/visumax-800.
* Data on file, myopia with optical zone 6.5 mm.
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development.
Contact for investors:
Sebastian Frericks
Head of Group Finance & Investor Relations
Carl Zeiss Meditec AG
Phone: +49 3641 220 116
Mail: [email protected]
Contact for the press:
Frank Smith
Head of Global Communications Ophthalmic Devices
Carl Zeiss Meditec Inc.
Phone: +49 3641 220 331
Mail: [email protected]
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704), which is listed on the MDAX and TecDAX of the German stock exchange, is one of the world's leading medical technology companies. The Company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The Company offers complete solutions, including implants and consumables, to diagnose and treat eye diseases. The Company creates innovative visualization solutions in the field of microsurgery. With approximately 4,823 employees worldwide, the Group generated revenue of ?2,089.3m in fiscal year 2022/23 (to 30 September).
The Group's head office is located in Jena, Germany, and it has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the USA, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, strengthen the Company's presence in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG's shares are in free float. The remaining approx. 59 percent are held by Carl Zeiss AG, one of the world's leading groups in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med
SOURCE Carl Zeiss Meditec AG
These press releases may also interest you
|
News published on and distributed by: