Subject: FDA
Tyber Medical Granted FDA Clearance on Distal Radius Plating System
Tyber Medical Expands Its Extensive Plating Portfolio with FDA Approval of Distal Radius Plating System for Fractured Radius, Ulna and Hand Bones
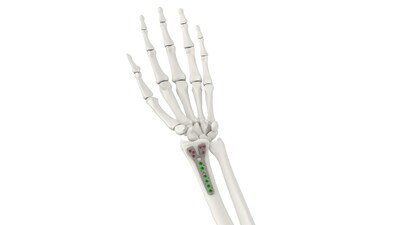
BETHLEHEM, Pa., Dec. 18, 2023 /PRNewswire/ -- Tyber Medical, LLC, a leading orthopedic device manufacturer providing private label orthopedic implants for the trauma, extremity, and spine markets, received a U.S. Food and Drug Administration (FDA) 510(k) clearance for its new Distal Radius Plating System. Tyber Medical's novel design covers a wide range of fractures, fusions, non-unions, malunions, or osteotomies of the radius, ulna, and hand. "The Tyber Medical Distal Radius System provides an innovative approach to address one of the most common fractures. Additionally, the enhanced ergonomic design delivers a solution to reduce flexor tendon irritation, a common post operative concern," said Tyber Medical CEO and President Jeff Tyber.
Tyber Medical's Distal Radius Plating System maintains stability and orientation in the anteroposterior plane utilizing a dynamic grouping of plate length options, offered in titanium or stainless steel. Tyber Medical's Chief Technology Officer, David Hannah, said, "our engineering team did a great job keeping the design under the watershed line while maintaining low prominence and the ability to reach the radial styloid. They recognized the need for an additional locking head size to accomplish this in the flexor tendon and narrow volar plates, and now we get to add that locking mechanism to our portfolio."
About Tyber Medical LLC
Tyber Medical LLC, is a leading orthopedic device manufacturer providing rapid access to FDA-cleared and CE-marked private label, portfolio-enhancing, regulatory-approved orthopedic implants for the spinal, extremity, and trauma markets. Tyber Medical provides customers with a quick and seamless path to market. Since it was founded in 2012, the company has released more than 50 spine, extremity, and trauma systems. Tyber Medical aims to develop and utilize differentiated technologies to make advanced orthopedic implants.
Contact:
Eric Dickson, Tyber Medical
Phone: 610-849-1710
Email: [email protected]
SOURCE Tyber Medical
These press releases may also interest you
|
News published on and distributed by: