Subject: FDA
Regenesis Achieves FDA Clearance for the New Reprieve by Regenesistm Device
Expands efforts into the podiatric space with a focus on the treatment of pain associated with diabetic neuropathy
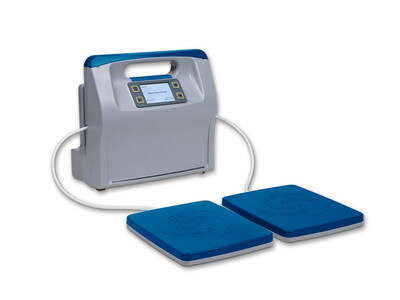
SCOTTSDALE, Ariz., Aug. 22, 2023 /PRNewswire/ -- Regenesis Biomedical, Inc., a medical device company focused on safe, non-drug, pain management, announced today commercialization of the new Reprieve by Regenesistm device (Reprieve). Reprieve is the only home-use shortwave diathermy device on the market and is based on Regenesis' core pulsed electromagnetic energy platform.
"In alignment with our Vision to improve health for a longer, more active, and fulfilling life, we are pleased to announce we received Food and Drug Administration (FDA) clearance for our new Reprieve by Regenesis device," said Tom Eisiminger, President and CEO. Commercialization is underway.
Eisiminger continued "Reprieve allows us to market our therapy for broad pain indications within the Department of Veterans Health Administration (VA), which represents a market that is 16X the size of our current market. Because our new device has a two-treatment applicator option, we are expanding our efforts into the podiatric space with a focus on the treatment of pain associated with diabetic neuropathy."
About Regenesis
Regenesis is a privately held medical device company dedicated to improving human welfare through the research, design, manufacture, and sale of energy-based medical products and services that alleviate pain, restore health, and improve quality of life. We focus on serving the needs of Veterans through our direct salesforce. Over 23,000 patients have treated with Regenesis' safe, non-drug, electromagnetic energy for pain management.
This press release includes forward-looking statements that reflect management's current views of future events. Actual results may differ materially from the forward-looking statements contained herein due to other important factors.
SOURCE: Regenesis Biomedical Inc.
SOURCE Regenesis Biomedical, Inc.
These press releases may also interest you
|
News published on and distributed by: