Subjects: PDT, FDA
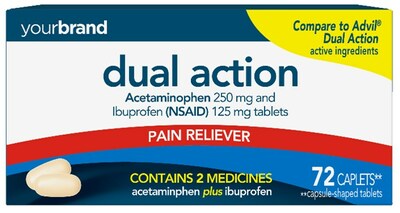
Perrigo Announces FDA Approval of the Store Brand OTC Equivalent of Advil® Dual Action Tablets
DUBLIN, March 1, 2023 /PRNewswire/ -- Perrigo Company plc (NYSE: PRGO), a leading provider of Consumer Self-Care Products, today announced that it has received final approval from the U.S. Food and Drug Administration ("FDA") for its Abbreviated New Drug Application ("ANDA") for Acetaminophen and Ibuprofen Tablets, 250 mg/125 mg, the store brand over-the-counter ("OTC") equivalent of Advil® Dual Action Tablets 250 mg/125 mg. The Company anticipates launching the product by Spring 2023.
Perrigo Executive Vice President & President, Consumer Self-Care Americas, Jim Dillard commented, "Following the successful approval and launch of Perrigo's first self-led branded prescription-to-OTC switch of Nasonex24HR®, this prescription-to-OTC approval further exemplifies the strength of our regulatory and development capabilities, enabling our business to quickly follow national brand innovation and consumer preferences. This approval was received on the first day possible following the expiration of the marketing exclusivity for Advil® Dual Action Tablets 250 mg/125 mg."
Acetaminophen and Ibuprofen Tablets, 250 mg/125 mg provides relief for multiple pain related symptoms by combining two ingredients indicated for OTC pain relief. Retail sales for the national brand equivalent product for the last twelve months ending January 29, 2023, were approximately $69 million based on IRI multi-outlet market data.
Important safety information about Acetaminophen and Ibuprofen Tablets, 250 mg/125 mg
Before using the product, consumers should read the Acetaminophen and Ibuprofen Tablets, 250 mg/125 mg drug facts label.
About Perrigo
Perrigo Company plc (NYSE: PRGO) is a leading provider of Consumer Self-Care Products and over-the-counter (OTC) health and wellness solutions that enhance individual well-being by empowering consumers to proactively prevent or treat conditions that can be self-managed. Visit Perrigo online at www.perrigo.com.
Forward-Looking Statements
This press release includes certain "forward-looking statements" within the meaning of the Private Litigation Securities Reform Act of 1995, as amended. Forward-looking statements relate to future events, such as future product sales and regulatory capabilities, and involve known and unknown risks, uncertainties and other factors?many of which beyond the Company's control?that may cause the actual results, performance or achievements of the Company to be materially different from its current expectations, assumptions, estimates and projections. Interested persons are urged to consult the Company's filings with the United States Securities and Exchange Commission, available at https://investor.perrigo.com/sec-filings, for a discussion of the Company's business and financial condition and certain material trends, risks, uncertainties and other factors relating thereto, including those discussed under "Risk Factors" in the Company's Form 10-K for the year ended December 31, 2022.
SOURCE Perrigo Company plc
These press releases may also interest you
|
News published on and distributed by: